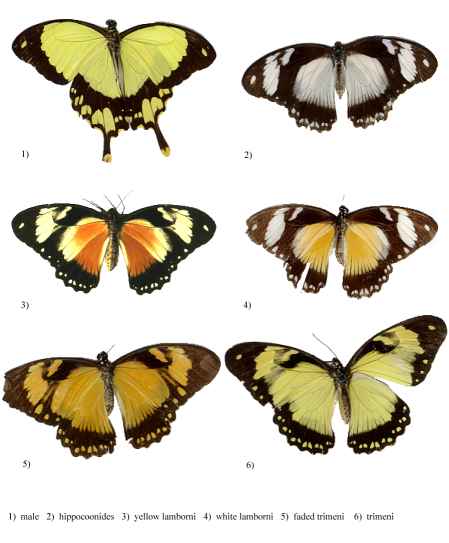
Figure 3-1 The different morphs of Papilio dardanus (race tibullus from Pemba, collected by Cook et al., 1994)

When studying visual signalling and perception in a species it is important to understand what visual signals they may be receiving from each other and from their surroundings in general. Butterflies have the greatest range of spectral sensitivity known for any animal. Some range from approximately 200nm (Ultra Violet) to 700nm (near Infra Red) (Bernard, 1979), as they often possess four visual pigments - sensitive in the red, green, blue, and UV regions. The 200-400nm UV range has been shown to be particularly important for butterflies, and UV markings overlay patterns in the Visible spectrum in many species (e.g. Silberglied, 1979; Brunton and Majerus, 1995). This is due to the two ways in which colours can be formed in butterflies - pigmental and structural.
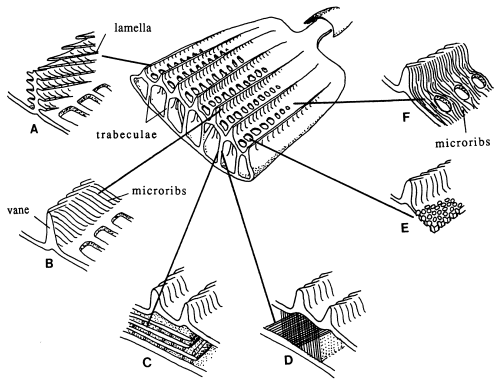
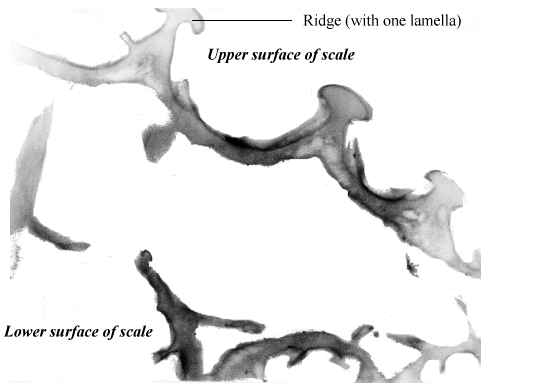
Butterfly wings are made up of a lower and upper membrane, both of which are covered in scales overlapping like roof tiles. Each scale can be coloured, and the overall colour of the wing is determined by the mosaic of coloured scales. These scales can contain different pigment chemicals which cause the colours that we can see in the Visible spectrum, and also occasionally in the UV range. UV light can also induce fluorescence in the Visible range, although this is usually comparatively very much weaker than colours produced by reflectance (Cockayne, 1924). In addition to the pigment which it contains, each scale can also have a 'structural colour' caused by the way its surface scatters incident light. Scale structure was first thoroughly studied by Mason (1926, 1927a, 1927b), and more recently has been studied in great detail by Ghiradella (Ghiradella et al., 1972; Ghiradella, 1974; Ghiradella & Radigan, 1976; Ghiradella, 1984, 1985, 1989, 1991, 1994). The scales are remarkably complex in design (see Figure 3-3), consisting of a smooth basement lamina and an upper lamina which is thrown into a series of ridges. The ridges are made up of stacks of lamellae, and these stacks are fringed by microribs.
Structural colours can be formed in a variety of ways. Ghiradella (1984, 1985, summarized in 1991) details six methods which have been identified. Most butterfly groups use only one or two of these methods, but the Papilionidae are unique in displaying all six (Ghiradella 1985). The most common method of producing a structural colour is for either the lamellae forming the ridges, or the microribs joining them, to be exaggerated, forming a stack of layers of chitin alternating with air. The exaggerated lamellae in the first of these methods give the ridges a characteristic 'Christmas tree' structure in cross-section (see Figure 3-3a). Light incident on these structures is reflected off each one in turn, giving rise to multiple thin-film interference (see introduction to Experiment 3-3 and also Land, 1972 for a description of this). The overall wavelength reflected depends on the number and thickness of the layers and the air-spaces between them. Examples of this form of iridescence are seen most spectacularly in the South American blue Morpho species (Mason, 1927a; Bingham et al., 1995); but with very fine lamellar spacing, UV reflectance can be achieved, as seen in Colias and Eurema species (Ghiradella et al., 1972). In some cases a pigmental coloration and an overlying structural coloration can be combined to form a different hue, such as metallic violets - blue iridescence combined with a red pigment (Nijhout, 1985).
The yellow pigment seen in males (and some females) of Papilio dardanus, Papiliochrome II (Umebachi & Yoshida, 1970), is known to fluoresce in the Visible region of the spectrum when illuminated with UV light (Cockayne, 1924). Other than this pigment, the colours found in this species appear not to have been studied in detail. Cook et al. (1994) reported that older males in the field became increasingly orange rather than yellow, and this accords with Cockayne's description (1924, p6) "This yellow readily changes to a browner tint and becomes non-fluorescent", but this potentially important age cue appears not to have been studied further at all. In Chapters 4 & 5, the colour perception of the butterflies is investigated with respect to their choice of flowers and mates, and so in this chapter their signal reception is studied - what signals are they actually likely to receive from each other?
In addition, in order to speculate which butterflies are mimics of which it is especially important to have an idea of how the butterflies may appear to their main predators. In this case the main predators are assumed to be birds, which also have a colour reception system which allows them to perceive UV coloration (e.g. see Chen & Goldsmith, 1986). Thus, for an assessment of the visual signals made by the butterflies to conspecifics and to predators, a quantitative measure of their patterns and coloration is required throughout their visual range (approximately 200-700nm). It is possible to do this in an accurate and quantitative manner using a spectrophotometer, but the method is complicated by the directional nature of the structural colours which occur in Papilio dardanus. These colours may be lost or inaccurately assessed if the spectrum is measured at only one angle to the direction of illumination (as was done in the analysis of the colours in Argynnis paphia in Experiment 3-3 where pigmental colours only were being studied). This can be overcome in one of two ways. The first is to measure the reflectance off the wings at a wide range of angles. This is practically quite difficult, and very time-consuming. It also produces a very large amount of data which is difficult to interpret. The second method is to use an integrating sphere, which captures and sums the reflection at every angle. Although the latter is a more robust method, it fails to resolve the directional component of the structural colours. If the structural colours are caused by the mechanism of thin-film stack interference, in which the light hits a stack of lamellae and is scattered by reflecting off each surface in turn (see Experiment 3-3 for a more detailed discussion of this) it should be possible to predict from measurements of the thicknesses and spacing of the lamellae how the structural colours would appear.
In order to understand butterflies' perception of colours it is necessary to gather quantitative data not only on the colours of the various areas of their wings, but also on their colour reception capabilities. The electrical response of retinal neurones to light of different wavelengths can be measured quantitatively (Autrum, 1958), and this allows an objective assessment of what the butterflies actually see. Only when this is known can real conclusions be drawn about any colour preferences they may demonstrate in behavioural experiments and therefore about their perception of colours.
The main aims of this investigation are:
From these results it is hoped to be able to:
In order to achieve these aims, the investigation was split into several parts. Specimens were firstly photographed under UV light to look for areas of UV reflectivity (Experiment 3-1). Then the fluorescence of the yellow pigment was quantified, and the other pigments checked for fluorescence with a fluorescence spectrometer (Experiment 3-2). In Experiment 3-3, the reflectance spectra of the pigments were measured using a spectrophotometer with an integrating sphere attachment. The structure of the scales was then investigated using both scanning (Experiment 3-4) and transmission (Experiment 3-5) electron microscopes and measurements of the lamellae and air spaces used to model the structural colours of the scales. Experiment 3-6 attempted to measure the colour reception capabilities of the butterflies in order to assess how they might see the spectra measured. Finally, Experiment 3-7 investigated the fading process which causes the yellow pigment in Papilio dardanus to become more orange with exposure to light.
This first part of the investigation of the visual signals presented by the butterflies is designed to determine the patterns present on the upper surfaces of the wings. It concentrates on determining whether there are any areas of the wings which reflect UV light, since in many species these are reported to have little or no correlation with the Visible pigmentation patterns (Silberglied, 1979). Hence there may be patterns, caused by UV reflectance, which are not visible to the human eye.
Cockayne (1924, p7) described the appearance of Papilio dardanus and its morphs under UV light, and reported that the yellow pigment in males and trimeni females fluoresced blue-green, that the white areas of hippocoonides fluoresced violet, and the other colours showed no fluorescence. The yellow pigment in the males has subsequently been identified and named as Papiliochrome II (Umebachi 1961). Cockayne does not report in any more detail the appearance of the different forms under UV light, and he did not photograph the specimens in order to assess UV reflection.
Since our own eyes cannot see into the UV region of the spectrum we are unable to assess whether or not there is a UV component to any coloration. However, monochrome film is sensitive to UV wavelengths, and hence by photographing a specimen lit purely by UV light using monochrome film it is possible to see which patches reflect UV light as they show up as bright areas on the print. Normal glass photographic lenses may filter out some UV light, but in view of the considerable cost of UV-transparent plastic lenses, the experiment was carried out using a glass lens. Since some UV does pass through the lens, long exposure times were used to compensate for the degree of filtering.
The aim of this experiment is to determine whether or not there are UV reflecting scales present in Papilio dardanus, and if so whether or not they form patterns other than those seen in the Visible spectrum.

Dead specimens of Papilio dardanus collected by Cook et al. (1994) from the island of Pemba, Tanzania, were used in this study. The specimens had been kept in dark conditions, protected from sunlight. Cook et al. describe this population of race tibullus as having three female morphs (see Figure 3-1): the yellow and black 'male-like female' trimeni, the black and white Batesian mimic of Amauris niavius (Trimen, 1868), hippocoonides, and the rare orange-tinged lamborni (also known as trophonius). Within the classification of lamborni there are two types - those which are similar to an orange-tinged trimeni ('yellow lamborni', or prototrophonius), and those similar to hippocoonides ('white lamborni', or trophonius) which is even rarer. In some races trophonius is a convincing mimic of Danaus chrysippus (Trimen, 1868) which is present on Pemba but on the island it is debatable whether the morph is a mimic or not. In addition, Cook et al. reported that the yellow pigment seen in the males and trimeni females, Papiliochrome II (Umebachi 1961), fades in sunlight to an orange colour (see Figure 3-3), and these 'faded' specimens were also studied.
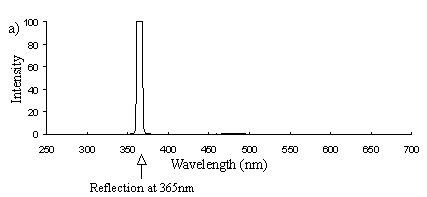
A Canon EOS 650 camera with a 55-200mm Sigma lens was set up on a tripod in a dark room, loaded with Kodak T-Max 3200 black and white film. The camera was mounted directly above the specimens, and photographs were taken of the butterflies placed on black velvet under three different lighting conditions: a fluorescent tube lighting the specimen from directly above, 254nm wavelength UV, and 356nm wavelength UV light, both supplied by a UV fluorescent tube with two alternative wavelength outputs placed adjacent to camera, illuminating the specimens from above.
Photographs of the morphs and of Amauris niavius and Danaus chrysippus taken against a black background were also captured in 32 bit colour using a Silicon Graphics IndyCam(tm) digital camera (Model CMB006C) under fluorescent tubes (illuminating from above) and 356nm UV light (placed above the specimens as before). Both the upper wing surfaces and the undersides of the wings of the Papilio dardanus specimens were photographed.
1) Using monochrome film
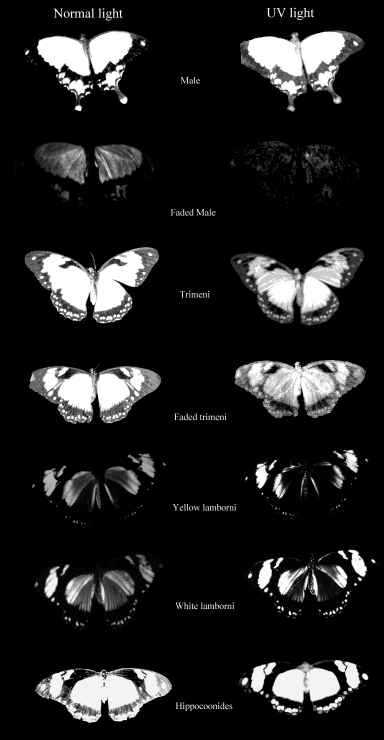
The photographs taken under 254nm light were identical to those taken under 356nm light except that the reflectance from them showed less intensely on the film. Only those under 356nm light are shown in Figure 3-4.
The yellow pigment of the males (Papiliochrome II) fluoresced a bright greenish yellow under both wavelengths of UV light, showing the same pattern as when illuminated with normal light. The tips of the tails, which are slightly orange coloured, did not fluoresce. Essentially the unfaded males looked very similar under the two light wavelengths.
The orange, faded males looked very different under UV light, hardly fluorescing at all, and gaining a 'speckled' appearance. The obvious black markings also faded, becoming hardly visible.
The trimeni females fluoresced similarly, although the forewings did not appear as bright. The black 'epaulette' markings near the base of the leading edge of the front wings became particularly obvious. It may be of significance that these epaulettes are present in the tailed, andromorphic females (but not the males) of races meriones, humbloti, and antinorii (Trimen, 1868).
The faded trimeni females lost a lot of their fluorescence. The black markings became less obvious, except for the epaulettes which remained dark. Around them some fluorescence remained, providing quite a lot of contrast, and the veins (particularly those on the hind wings) also fluoresced, unlike those on any of the other morphs (including the faded male). This could be due to scales around the veins being slightly more protected from damage than the others on the wing.
The yellow lamborni females appear to have the same Papiliochrome II pigment and markings as trimeni females. They also have a bright orange pigment on the hind wings and the lower fore wings. This orange pigment absorbs UV light, appearing as dark as the black markings in the photographs.
The white lamborni females have white patches in the place of the yellow on the yellow lambornis, and the black epaulette markings are more continuous with the wing edgings and sharply defined as in hippocoonides. The orange pigment is slightly paler than that in yellow lamborni, and the white patches have a pale pinkish sheen to them. Under UV light the orange pigment absorbs (although possibly not as much as that in yellow lamborni), and the white scales were not observed to fluoresce as described by Cockayne (1924). However, on the film, the white patches appear as bright as the yellow pigment on the other morphs, indicating that it is reflecting, but in the UV spectrum where it could not be observed. Another piece of evidence that these bright regions are due to UV reflectance is the blur on their edges - particularly noticeable on the image of hippocoonides (see Figure 3-4). This is due to the fact that the camera was focused using Visible light, whilst UV light has a shorter wavelength. It is possible that the UV light which Cockayne used included some visible wavelengths, and it was these violet wavelengths being reflected by the white patches that he described as violet fluorescence. He describes this violet fluorescence in a lot of species with white markings, and it seems likely that these are in fact reflective to all wavelengths rather than being fluorescent.
Hippocoonides females have similar markings to the other females, although the black patterning tends to be less variable, and resembles that of their model, Amauris niavius. The background colour is white, with a pinkish sheen (as is Amauris niavius). Under UV light neither the model (see Figure 3-5) nor the females themselves appear to fluoresce, but on film the white appears bright, again suggesting that the scales reflect UV light.

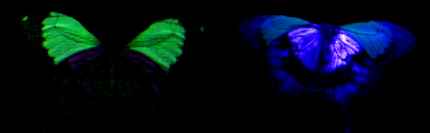
2) Using a digital camera
Before undertaking this experiment, it was not known whether or not the digital camera would be sensitive to UV light, but the images obtained (see Figure 3-5) show the white patches on hippocoonides and white lamborni even more strongly than the yellowish fluorescence from Papiliochrome II. All the photographs taken under UV light have had their luminescence increased by 200% (using Paint Shop Pro version 4 on a PC). As before, the white patches did not fluoresce visibly, and so the camera must have been sensitive to some UV light reflected, and displays this as a purple coloration. These images are probably similar to what Cockayne (1924) observed. It is noticeable, however, that the purple coloration of the white patches under UV light is 'shiny', rather than being of a uniform colour and intensity as seen in the fluorescence of the male. This indicates that the coloration observed is due to reflection rather than fluorescence. Experiment 3-2 should confirm whether or not this is indeed reflection rather than fluorescence. It is also interesting that the trimeni female appeared not to fluoresce as strongly as the male. Cockayne (1924, p7) described the trimeni females as fluorescing "with the same brilliant blue-green colour as the male" so it could be that this particular individual was beginning to fade and lose fluorescence. This is investigated further in Experiment 3-2. The photographs of the undersides of the wings reveal that white patches on the underside of the wings of hippocoonides reflect UV just as those on the upper surfaces do. The same is true of the white patches on the undersides of the forewings of the white lamborni morph. Both the yellow patches on the forewings and the orange cryptic hindwings of trimeni and yellow lamborni, appear to reflect the UV but not to fluoresce. The faded male also appears to show UV-reflecting hindwings, whilst the unfaded male appears very different - with the yellow forewings fluorescing and the hindwings neither fluorescing or reflecting UV. However, photographs taken of other male specimens (at slightly different angles of incidence of light due to the fact that the specimens were not entirely flat, and inclined to rock slightly when placed on their upper surfaces) reveal that sometimes reflectance is observed on both fore and hindwings, and sometimes only fluorescence on the forewings is seen(see Figure 3-6). This is probably due to the fact that the reflectance is only visible at some angles, and that at some angles its presence on the forewings makes the fluorescence invisible. This suggests that the fluorescence is much less intense than the reflectance, which will be tested in Experiment 3-3. It is interesting to note that although the yellowy orange undersides of the hindwings of the males and trimeni females do not fluoresce, they do reflect UV light. This will alter their appearance to predators with UV vision compared with how we perceive them.
It can be seen that the white scales in hippocoonides and white lamborni females reflect UV light and that the Papiliochrome II pigment found in males, which fluoresces a greenish yellow under UV light, also appears to be found in both trimeni females and yellow lamborni females. The orange and black pigments do not fluoresce and the scales do not reflect UV light. It is difficult to tell whether or not the scales containing the yellow fluorescent pigment also reflect UV light, since such a reflectance might be masked in the pictures by the fluorescence. The monochrome film only records the intensity and not the wavelength of the light involved. In the colour photographs, the fluorescent patches appear yellow when the photograph is taken from directly above. However, the evidence from the photographs of the undersides of the wings taken at a slightly oblique angle indicates that directional UV reflectance may be present in the yellow patches, but merely masked at that particular angle by the fluorescence. Experiment 3-3 investigates the presence of UV reflectance further.
| 'Human colour' | Morphs found in | UV reflectant? | Fluorescent under UV? |
|---|---|---|---|
| Black | All | No | No |
| White | Hippocoonides, White lamborni | Yes | No |
| Yellow | Male, Trimeni, Yellow lamborni | Probably | Yes |
| Bright Orange | White lamborni, Yellow lamborni | No | No |
| Yellowy orange | Underside of hindwings of all but hippocoonides | Yes | No |
Given that the UV reflectance is almost certainly achieved by structural means, from these pictures it would be predicted that the scales in the black and orange areas would have a different structure from those in white areas, lacking reflective structures. It is not possible to predict from these pictures whether or not the scales containing yellow pigment also possess UV reflective lamellae.
These photographs also indicate that there are no patterns due to UV reflectance which are uncorrelated with the Visible patternings. This means that the butterflies should appear similar in patterning to each other and to their predators as they do to us, but that their coloration is likely to be seen very much differently due to the presence of UV reflectance which is invisible to humans. Experiment 3-3 quantifies the colours used by these forms, and Experiment 3-6 quantifies the visual sensitivities of the butterflies in order to assess how the colours might appear to them.
Since Papiliochrome II, the yellow pigment in Papilio dardanus, fluoresces in the Visible spectrum under UV light, any spectra produced of this pigment must take this into account as in daylight, the fluorescence will add to the colour produced by the yellow patches. For example, if a spectrophotometer emits each wavelength of light separately and then measures only how much of that particular wavelength returns from the wing surface, this fluorescent component will be missed. In order to add the component back in, therefore, it is necessary to measure the wavelength emitted and its intensity at each wavelength of incident UV light. This can be achieved using a fluorescence spectrometer, which, unlike a traditional spectrometer, has two monochromators - one to produce the incident beam and the second to monochromate the reflected light. Thus the wavelengths of emitted light are recorded as well as their intensity.
This method should also clarify whether or not the violet 'fluorescence' recorded by Cockayne (1924) as being emitted from the white patches in hippocoonides under UV light was fluorescence or reflectance of wavelengths in the Visible region of the spectrum not filtered out by his apparatus.
The aim of this experiment is to determine the wavelengths of light which produce fluorescence in Papiliochrome II, and both the wavelengths and approximate intensities of the fluorescence produced in order to assess the impact of the fluorescence on the overall reflectance spectrum of the coloured patches. It is also the aim to determine whether or not the white patches in hippocoonides fluoresce.
A Perkin Elmer LS50 fluorescence spectrometer was set up so as to allow a mounted butterfly to be placed into the excitation beam, and a spectrum of the resulting scattered light to be recorded and plotted on a PC. Whole specimens were pinned onto non-reflective, matt black polystyrene, and then placed so that the beam of incident light formed a focused band (approximately 1mm x 1cm) on the appropriate area of the wing. Two types of scan were used:
Emission scans were performed on the main pigments in each morph, and also the black from a male. Where a pigment was found to fluoresce, an excitation scan was done at several wavelengths throughout the fluorescence band.
1) Emission scans (see Figure 3-7 and Figure 3-8).

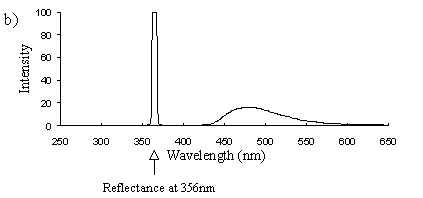
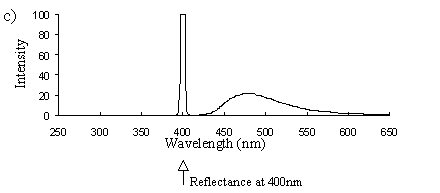
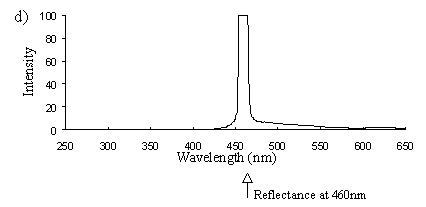
Emission scans of the Papiliochrome II pigment found in males were done at 257nm, 356nm, 400nm, and 460nm. At all these wavelengths a broad fluorescence band was seen between 425nm and 630nm, with a peak at 483nm. In each graph the high peak is the scatter from the wavelength applied or its 1st harmonic (at double the wavelength).
The same scans were done on the yellow pigment in trimeni and yellow lamborni females, and the same fluorescence was seen, and at the same intensity.
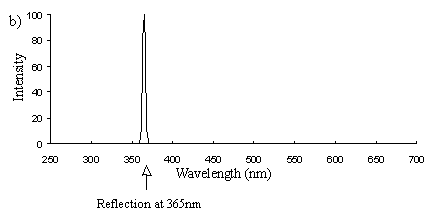
Emission scans at the same wavelengths of the sunlight-faded Papiliochrome II of males showed no fluorescence at all, the only peaks being those due to scatter of the incident light (Figure 3-23a).
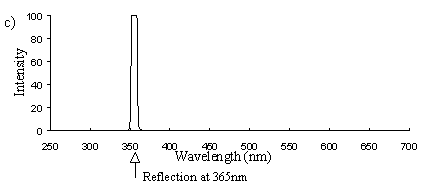
The orange pigment in yellow lamborni showed no fluorescence (Figure 3-23b). There is also a suggestion that it reflects this wavelength less well as the reflectance peak appears not to be as large, but this is investigated fully in Experiment 3-0.
Emission scans at the same wavelengths of the white scales in hippocoonides also showed no fluorescence (Figure 3-23c), and the same was true of its model, Amauris niavius.
2) Excitation scans (see Figure 3-9) Excitation scans of the yellow pigment showed that at the peak wavelength of fluorescence (483nm) the excitation wavelengths formed a smooth, shallow curve from 340nm to a peak at around 400nm and then dropped off more rapidly from 420 to 440nm.
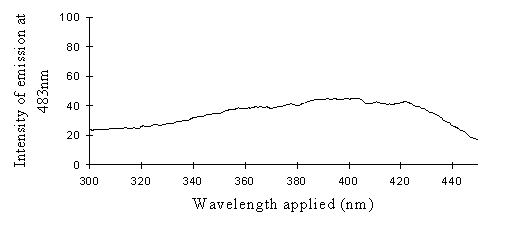
The fluorescence spectra of the yellow pigment were found to be the same in males and the trimeni and yellow lamborni females. This pigment, Papiliochrome II (Umebachi, 1961) is reported to have a peak of absorption at 380nm (Umebachi & Yoshida, 1970). Here it is found to fluoresce at a range of wavelengths around 483nm when exposed to UV light of wavelengths between 340nm and 400nm (the wavelengths which it absorbs most readily). The fluorescence is, however, very weak when compared with the reflectance of the wings (seen in Figure 3-7), so the fluorescence of the pigment will have a negligible effect on the overall colour of the yellow wing patches. This fluorescence is lost in older specimens where the yellow pigment has faded to orange, but the weakness of the fluorescence means that this loss will not significantly contribute to the colour change of the patches. The breakdown of Papiliochrome II in sunlight is examined in greater detail in Experiment 3-4.
The white patches of hippocoonides were found not to fluoresce when exposed to UV light of any wavelength. Thus it can be concluded that the violet 'fluorescence' reported by Cockayne (1924), and the colours noted in Experiment 3-1 were due to reflectance of the incident light rather than fluorescence of a pigment.
Reflectance spectra are an objective measurement of the colour of an object. This is very important in the study of visual signalling as different species have different spectral sensitivities. For example, two butterflies may be indistinguishable to humans, but easily separable by animals which see in the UV region of the spectrum due to a UV component in the colour of one of the butterflies. Measuring the reflectance spectra of the colours allows humans to measure exactly what visual signals are being produced and received by the butterflies, and thus to assess the similarity of species, especially in the case of comparing potential model and mimic pairs. As discussed in the introduction to this chapter, the directionality of structural colours in butterflies complicates the process of measurement. Simply measuring the reflectance at one angle relative to the angle of illumination can misrepresent the total reflectivity of the scales, as their structure reflects different wavelengths at different angles relative to the illumination. Although it is technically possible to measure the reflectance from the wing at every angle, this is very laborious and produces a very large amount of data which is difficult to interpret. In this case the problem may be simplified by taking measurements using an integrating sphere attachment to the spectrophotometer, which collects reflected light from every angle and records only the total reflectance. Microscopy of the scales can reveal how the structural colours are formed, and some of these methods can be modelled mathematically to predict the exact form of the structural colours.
The aim of this experiment is to achieve an objective measurement of the coloration of the various morphs of Papilio dardanus in order to assess how they might appear to each other and to their predators.
A Shimadzu UV- 2100 Spectrophotometer with integrating sphere attachment was used to measure the reflectance spectra of the colours. The wing samples were held against the 18mm diameter circular sample hole in the integrating sphere by a matt black, spring-loaded mount. The central area of the sample (5.5mm x 3mm) is used.
Two beams enter the sphere alternately, one from either side. One is directed at a totally reflective BaSO4 control, the other at the sample. The beams hit the surfaces at 8( to the normal, and all the scattered light is then gathered by the internally reflective sphere and measured by a photomultiplier at the base. The intensity of the reflection of the sample is plotted at each wavelength as a percentage relative to the reflectance of the control.
The spectrophotometer measures the reflectance at each wavelength in turn, at 0.5nm intervals, from 240nm to 800nm. A different bulb is used from 360nm downwards into the UV, sometimes causing a slight discontinuity in the spectrum at 360nm. The results are plotted on a PC, and can then be exported in ASCII format to a spreadsheet for analysis.
Scans were done of the yellow coloration on the upper surface of the forewing, lower surface of the forewing, and upper surface of the hindwing of three male specimens of races tibullus, cenea, humbloti, and one male of meriones of Papilio dardanus. This allows a comparison of the different areas of the wing, all of which are thought to contain the same pigment, but which might have differing structural colours. It also compares the different geographic races of the species (See Appendix 1 for a summary of the races). Little is known about the inter-relatedness of the races, and particularly it is not clear which most represents the primitive condition (either the polymorphic land races, or the monomorphic island races meriones and humbloti). Any racial differences in the colour may therefore be phylogenetically useful.
Scans were next done of the yellow coloration on the upper forewing, lower forewing, and upper hindwing of three specimens of the trimeni female morph from race tibullus, and a female specimen of race meriones of Papilio dardanus. Any differences between the spectra of the andromorphic females, and between them and the males may shed light on whether the tailed females in meriones are ancestral or derived, and how closely the tail-less andromorphic females resemble the males in colour.
Scans were next done on the yellow patches on the upper forewings of three specimens of the yellow lamborni female morph from the tibullus race of Papilio dardanus. It is likely that this morph has evolved from the trimeni morph, and if this is so, the colour of the yellow patches on the wings should match that of the trimeni females.
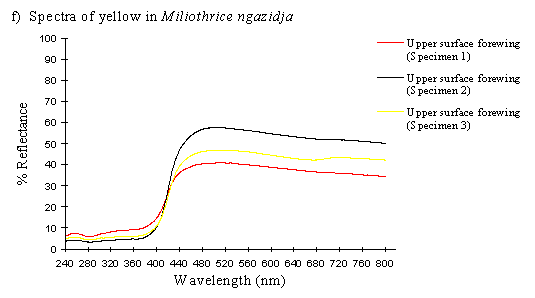
To contrast these results, scans were done on the upper forewing of three specimens of Mylothris ngaziya, a butterfly from Grande Comore. This butterfly appears, to human observers, very similarly coloured to the Papilio dardanus of race humbloti that fly in the same areas. Since Mylothris ngaziya is generally considered unpalatable (since Carpenter, 1941), it is possible that the Papilio dardanus may be gaining some protection from mimicry of it. These scans should reveal whether or not the two species have genuinely similar spectral colours.
Scans were made of the upper surface of the forewings of three old and faded specimens of the female morph trimeni and one faded male from the tibullus race of Papilio dardanus. The colour change of the yellow patches reported by Cook et al. (1994) has not been quantified before, so these scans should reveal exactly what changes are taking place.
The spectra were obtained for the orange pigment from the upper surfaces of the hindwings of three specimens of each of the white and yellow lamborni females from the tibullus race of Papilio dardanus, and also for the orange pigment from one specimen of Danaus chrysippus. Cook et al. (1994) hypothesised that the lamborni females could be mimicking the old, faded males (which appear orange) in order to avoid sexual harassment from males, whilst others (originally from Trimen, 1868) conclude that lamborni is a mimic of the butterfly Danaus chrysippus. The analysis of the spectra of the orange pigments may clarify this issue.
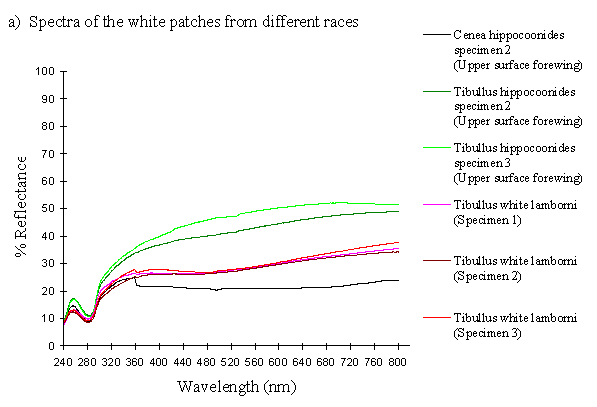
The white coloration on the upper surfaces of the forewings and hindwings of 3 specimens of the hippocoonides female morph from race tibullus and 2 specimens from race cenea of Papilio dardanus were analysed, and compared with the white pigment from the undersides of the hindwings of 3 specimens of hippocoonides from tibullus and 2 from cenea race of Papilio dardanus. This should give a cross-race comparison of any variation in colour, and also determine whether there is any difference in the colour of the upper and lower surfaces of the forewings. Scans were also done of the white patches on the forewings of the white lamborni morphs from race tibullus. Comparison of these with the white patches in hippocoonides may shed some light on whether the white lamborni morph is likely to have evolved from a hippocoonides morph through the addition of orange pigment to the hindwings, or from a trimeni morph via yellow lamborni.
To compare with the data from the hippocoonides females, scans were done on the white coloration of the upper surfaces of the forewings of 3 specimens of Amauris niavius, and the bottom surface of one of the specimens (the others were too delicate to allow this). Hippocoonides is generally regarded as a mimic of Amauris niavius (Trimen, 1868), and these scans should indicate whether the two truly resemble each other in colour as well as pattern.
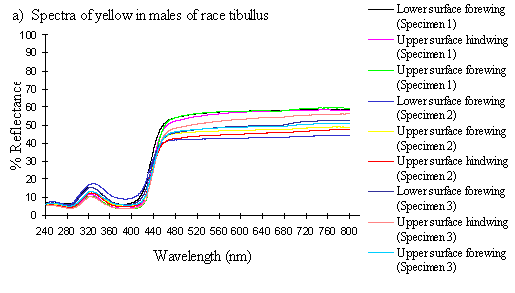
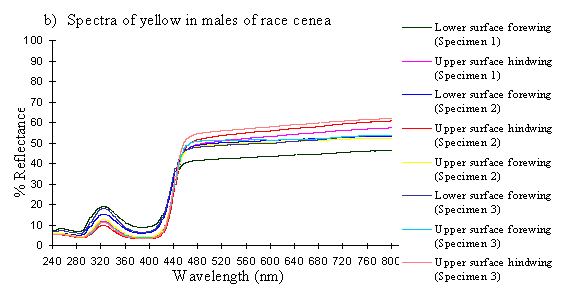
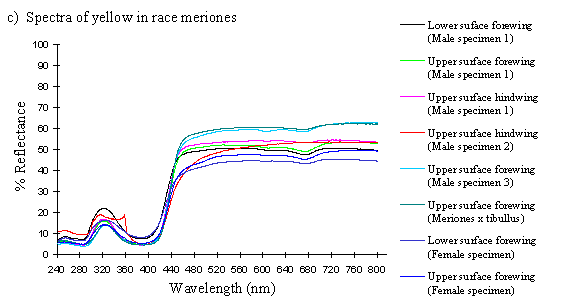
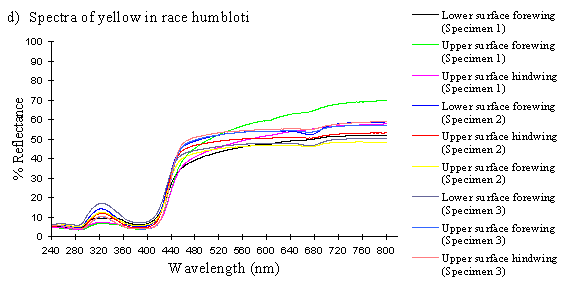

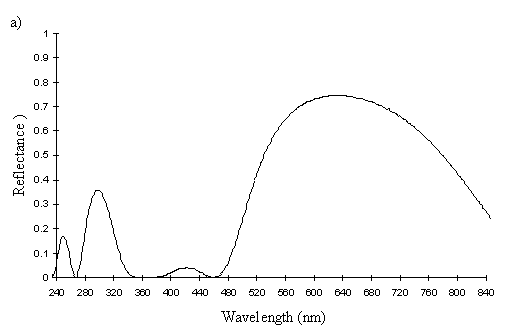
The reflectance spectra of the yellow pigments in all males (Figure 3-10a, b, c, and d), trimeni females (Figure 3-10e, blue-green lines), and yellow lamborni females (Figure 3-31e, red-pink lines) of Papilio dardanus were all very similar. In each case the reflectance was high (around 50%) from 800nm to about 445nm (red, yellow, and green). They all then show a sharp decrease in reflectance (to under 10%) over the blue/violet region of the spectrum until about 370nm, where the reflectance slowly increases to a small peak (under 20% reflectance) at 320nm in the UV region. The reflectance then returns to under 10% from about 290nm onwards (a slight fluctuation in the reflectance below this was also present in the control, empty, scan). The specimens (including the female) from the meriones (Figure 3-31c) and humbloti (Figure 3-31d) races show a slight dip in the reflectivity at about 680nm which was not seen in the races tibullus (Figure 3-31a) and cenea (Figure 3-31b). This might be a clue that these two races are closely related, and it is possible that an analysis of the pigments in the two sister species (Papilio constantinus and Papilio phorcas, Clarke et al., 1991; Vane-Wright & Smith 1991) might indicate whether this spectral feature is primitive to the species. If this is so then it would add further weight to the argument that these two monomorphic races represent the primitive condition for the species (Trimen, 1869 and subsequently Poulton, 1924; Ford, 1936; Clarke & Sheppard, 1963; Turner, 1963; O'Donald & Barrett, 1973; Clarke et al., 1985).
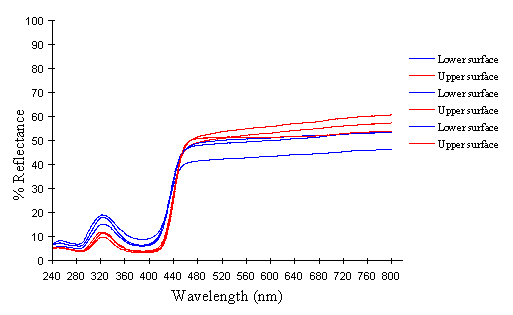
The spectra from the lower surfaces of the wings (even in trimeni females, Figure 3-31e) show the same reflectivity (although tending to be a little less reflectant) over the Visible spectrum, but the UV peaks are very slightly higher, showing a slightly higher reflectivity to UV light than the upper surfaces (see Figure 3-11). This would make them appear a very slightly different colour (more shifted to the blue end of the spectrum) than the upper wing surfaces to an animal with colour vision which included UV.
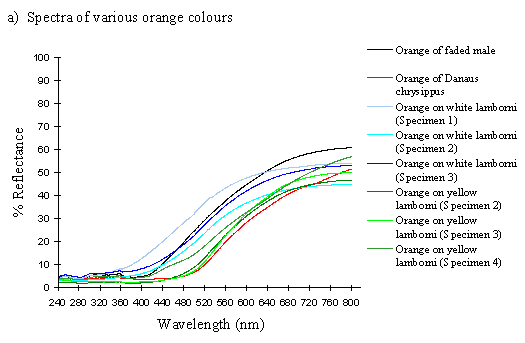
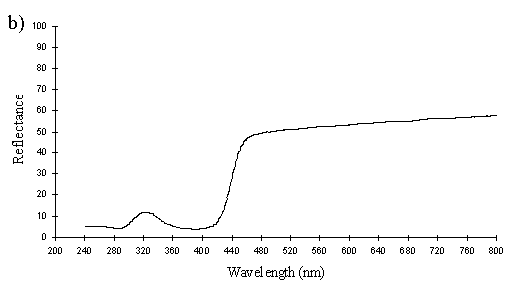
The spectra of Mylothris ngaziya (see Figure 3-31f) show that the pigment would easily be distinguished from that in Papilio dardanus by animals with UV vision. The reflectance rises slightly from about 40-50% at 800nm until about 480nm, when it starts to decline, steeply from 440nm until 400nm. From 400nm downwards the reflectance is very low (under 10%), markedly different from all the Papilio dardanus yellow spectra. This indicates that Papilio dardanus is very unlikely to be gaining protection from predators with colour vision including the UV through mimicry of Mylothris.
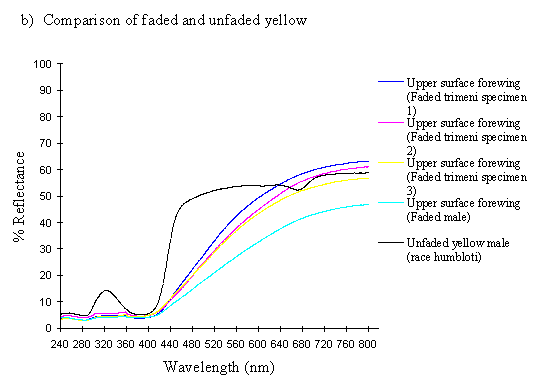
The spectra of the faded yellow pigment show a dramatic change from those of the pigment when fresh (see Figure 3-12b). The reflectance at 800nm is the same (around 50%), but this slowly decreases to about 5% at 410nm, and remains this low throughout the UV range. Thus the reflectance is high through the red and quite high in the yellow, but is increasingly low through the green part of the spectrum, and lacks any reflectance in the blue and UV. This explains why we see a gradual reddening of the pigment, as it loses reflectancy of green (which, combined with red causes us to perceive yellow), and to a lesser degree reflectancy of yellow. As Figure 3-12b demonstrates, the faded yellow shows a dramatically different spectrum from that of the unfaded yellow (shown in black), losing the green/yellow and UV reflectancy which gives the unfaded yellow such a characteristic shape.
The spectra of the orange patches in yellow and white lamborni specimens from the tibullus race of Papilio dardanus are very similar to each other, and show essentially the same sort of smooth curve as in those of the faded yellow pigment (see Figure 3-12a). The curve of the orange in yellow lamborni however (shown in green in Figure 3-12a), is a little more sigmoidal, with even less reflectance in the green part of the spectrum. This makes them a little redder in appearance to us. They all lack reflectance in the UV range (as was also demonstrated in Experiment 3-1).
The spectrum of the orange pigment in Danaus chrysippus is again a similar smooth curve (see Figure 3-12a) and is almost indistinguishable from the spectra of the orange patches in yellow lamborni.
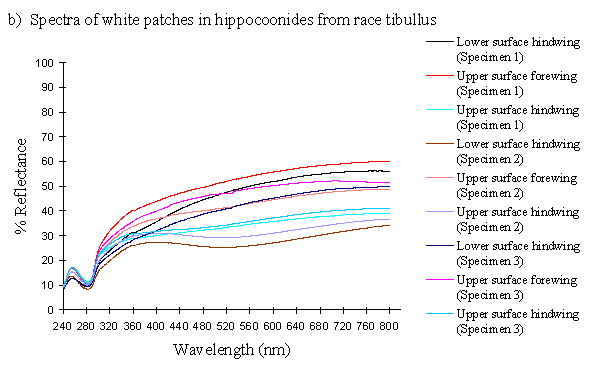
The reflectance spectra of the white patches in the hippocoonides and white lamborni morphs of Papilio dardanus show a basically high level of reflection throughout the Visible spectrum, as would be expected, but the hindwings of hippocoonides have a lower reflectance over the Visible range (see Figure 3-13b, the hindwings are plotted in blues, and the forewings in reds/pinks). All individuals show a considerable drop in reflectivity between about 360nm and 280nm, where the reflectivity is consistently 10% (▒2%). From here it again rises sharply to a small peak (between 10 and 20% reflectivity) at 250nm, and again drops off. Although the intensity of the reflection is variable, the shape of the curves in the UV region is extremely consistent. In the Visible spectrum there appear to be two possible patterns. Most specimens start with a high reflectivity at 800nm (30-60%) which slowly decreases in a steady curve until around 360nm, where the drop-off becomes steeper. A few others start between 20-30% reflective at 800nm and then decrease only very slightly, before either dropping off at 360nm, or rising from about 440nm to 360nm and then dropping. The specimens which tend to show this pattern are those from race cenea, or the white lambornis from race tibullus (see Figure 3-13a) although this difference could merely be due to the poor condition of these specimens (note in Figure 3-13b that the hindwing of specimen 2, from race tibullus, also shows this pattern). The lower surfaces of the wings show the same reflectance from 800nm until 360nm, but from then on the reflectance tends to be slightly lower than that of the upper surfaces, although the reflectance follows exactly the same pattern (see Figure 3-13b where the lower surfaces are shown in dark colours). This difference is very slight, and probably falls within the range of individual variation.
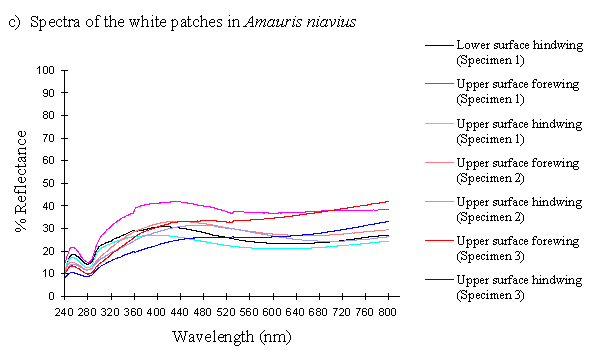
The spectra obtained from specimens of Amauris niavius are very similar to those of the white patches in Papilio dardanus (see Figure 3-13c). They tend to follow the second pattern described above in the Visible region, with a slight decrease in reflectivity in the middle of the region and then a slight rise followed by a sharper drop in reflectance from 360nm until 280nm. In the UV region the pattern of reflectivity is exactly the same. Again, there is no discernible difference between the upper and lower surfaces of the wings.
The yellow pigment in males, trimeni females, and the yellow patches of yellow lamborni (prototrophonius) females all appear to be the same (Umebachi & Yoshida's Papiliochrome II), although the slight difference in spectrum found from the island races humbloti and meriones may suggest that these two races are more closely related to each other than they are to the mainland races. The similarity of the yellow patches in all morphs which possess them means that little can be inferred from these results about the evolution of the various forms.
The spectra of the pale yellow wings of Mylothris ngaziya lacked the UV component found in the yellow patches of Papilio dardanus, indicating that to a predator with colour vision including UV the two species would not appear similar.
Papiliochrome II was shown in Experiment 3-2 to fluoresce weakly in the Visible spectrum (between 420 and 630nm) under a wide range of UV and violet wavelengths (from 340nm to 440nm with a peak at about 400nm). Its reflectance spectrum shows that it is highly reflective to red, yellow and green light (800-450nm), and the scales containing Papiliochrome II are also reflective to UV light with a wavelength around 320nm. The absorptive region from 360-420nm is in accordance with the maximum absorption wavelength of the pigment, which is described by Umebachi and Yoshida (1970) to be at 380nm. This also matches the wavelengths which most excite fluorescence, seen in the excitation scan of the pigment with fluorescence at 483nm in Figure 3-9, as demonstrated in Experiment 3-2. After prolonged exposure to sunlight, the yellow pigment loses its fluorescence and reflectancy to green light, appearing orange. In addition, the UV reflectancy of these scales is lost. The bases for both these phenomena are investigated further in Experiments 3-4, 3-5, and 3-7.
Experiment 3-2 demonstrated that there was no pigment present in the white patches of hippocoonides which fluoresced under UV light, but the patches are highly reflective of Visible and near UV wavelength light (800nm - nearly 300nm) and also reflective of UV light with a wavelength of about 255nm. The white patches in Amauris niavius show very similar properties. The spectra indicate that the hippocoonides morph of Papilio dardanus is indeed likely to be a convincing mimic of Amauris niavius although it is worth remembering that the integrating sphere collected light reflecting from all angles, and thus the two apparently similar spectra may have had very different angular components. Technically (Mason, 1926), true whites are all structural colours - an unpigmented cuticle will appear white due to the scattering of light by surface irregularities and air bubbles within the structure, and pigmental whites can be argued to be structural because they result from the scattering of light by colourless pigment granules. The small UV peak in both species is likely to be a structural colour caused by a more regular structural arrangement, and if this is so, then the fact that the peak in hippocoonides so closely matches that in Amauris niavius and yet is different from that in the yellow trimeni females suggests that hippocoonides has not evolved as a mimic simply through the loss of pigmentation in the yellow scales, but has actually changed the structure of the scales themselves to produce a sophisticated mimicry of the structural colours in Amauris niavius. Experiments 3-4 and 3-5 study the microstructure of the scales to investigate this further. It is interesting, however, that the small observed peak is so far into the UV range of the spectrum, as this is likely to be on the edge of the visual range of most animals, including the butterflies' presumed major predators, birds (see Chen & Goldsmith, 1986). The fact that the hippocoonides spectrum so closely matches that of Amauris niavius in this region suggests that it is either under selection by predators in this region, or that this small peak is simply a consequence of similarities in the more general structure of the scales (e.g. that all unpigmented scales have a similar spectrum). This should also be clarified by microscopy in Experiments 3-4 and 3-5.
The orange pigment found in the lamborni morphs does not fluoresce (as shown in Experiments 3-1 and 3-2), and shows a steady decrease in reflectancy across the Visible spectrum, and no reflectance of UV light. There is a very slight difference in the shape of the spectra from white and yellow lamborni females, with the latter showing a slightly sharper drop-off in reflectancy in the green region of the spectrum. The spectra from the orange in white lamborni specimens appears to be closer to that of the faded Papiliochrome II pigment, and that of the yellow lamborni specimens to be more similar to the properties of the orange pigment in Danaus chrysippus. It is thus very difficult to say from this evidence whether the lamborni morphs are convincing mimics of either.
The similarity between the white patches in hippocoonides and those on the forewings of the white lamborni morphs is intriguing. If indeed the hippocoonides morph has changed the structure of its scales in order to mimic Amauris niavius closely, then either the white lamborni morph has secondarily also changed the structure of its scales from that found in trimeni and yellow lamborni in order to mimic the white spots on the forewings of Danaus chrysippus, or it has evolved directly from the hippocoonides morph. It is unfortunate that the small size of the white spots in Danaus chrysippus made it impossible for their spectra to be analysed, as it is not known whether or not they share the same spectral shape as the whites in Papilio dardanus and Amauris niavius, and hence whether the white lamboni morph is indeed mimicking the colour of the spots. Again, a study of the microstructure of the scales should help clarify whether the similarity in the spectra of the whites is important or not.
In general, then, the white of hippocoonides and the white lamborni (and also of Amauris niavius) has been shown to be a 'true white' - reflectant to both light in our Visible spectrum and UV light. It is not yet known to what extent Papilio dardanus can detect light as far into the UV region as 200-300nm, so it is not yet known whether the reflectancy in this area will affect their perception of the colour. This is investigated in Experiment 3-6.
Most vegetation does not reflect UV wavelengths, and is dominated by wavelengths greater than 450nm (Klein, 1978; Menzel, 1979), and hence appears to us as it would to animals with a visual spectrum including both the Visible spectrum and extending into the UV. The spectral sensitivities of Papilio dardanus are investigated in Experiment 3-6 to determine how the morphs might appear. However, the white morph will be distinct from the background by nature of its 'brightness'. Although it has been argued (Kevan et al., 1996) that insects cannot detect white against a vegetation background due to the fact that they cannot detect differences in brightness, this argument is negated by experiments which clearly show that butterflies can (Swihart, 1971; Scherer and Kolb, 1987). However, the yellow trimeni females might not be so obvious, as their UV reflectance would cause their overall perceived colour to an animal with UV sensitivity to be pulled down slightly towards the blue end of the spectrum, probably into the green region. Their brightness may also be comparable to that of the background. It is possible, therefore, that the intense yellow that we perceive is actually far less obvious against a green vegetation background to animals with UV sensitivity than might be expected.
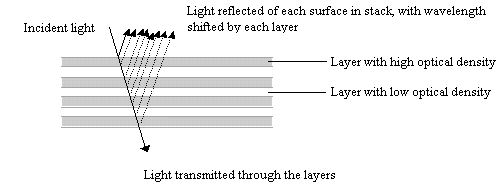
Butterfly scales have been studied extensively using electron microscopy, and their general structure is well known (see Figure 3-2, and also Ghiradella 1991 for a summary). The ridges described in the introduction to this chapter are separated by horizontal crossribs in most species, but in Papilionids this is replaced by a network of fine tubes. Ghiradella has described six mechanisms by which structural colours may be formed, all of which are illustrated by Papilionid species (Ghiradella, 1985, and again see Figure 3-2), but most of these rely on the general method of structural colour formation of thin-film interference. The optical effect of four of the six methods (methods A-D in Figure 3-3) can be approximated as a multiple thin-film interference filter (Land, 1972), in which light is reflected off each surface in turn through a stack of layers with differing optical thickness, and interference between the reflected wavelengths gives rise to an iridescent structural colour (see Figure 3-14 and Land, 1972 for greater detail). Thus spectral components arising from structural colours may be predicted using this model (eg. Anderson & Richards, 1942; Lippert & Gentil, 1959; Ghiradella et al., 1972) when the number, thickness and spacing of the layer-forming structures is known.
In Experiments 3-1 and 3-3, UV reflectance was demonstrated from the white and yellow scales of Papilio dardanus, and also from the white scales of Amauris niavius. By investigating the structure of the scales using Scanning Electron Microscopy (SEM) it should be possible to compare the microstructures of the scales of different colours, and also compare scales of Papilio dardanus with those of Amauris niavius and Danaus chrysippus, two of its putative models (Trimen, 1868). It is hoped that this study of the microstructure of the scales will confirm that the UV reflectance is indeed due to structural reflectance. SEMs have been obtained at a high enough resolution to measure the widths and spacings of lamellae (for example see Bingham et al., 1995), and so if thin-film interference is the mechanism producing structural colours in any of the scales studied, it may be possible to predict these using a mathematical model of this mechanism, and compare these predictions with the measured UV reflectance from the scales to confirm that this reflectance has a structural origin.
If the UV reflectance does indeed have a structural origin, it is hoped that the microstructure of the scales might elucidate whether the hippocoonides morph has truly evolved to mimic the UV reflectance of Amauris niavius so closely (see Experiment 3-3) or whether this is merely a coincidental similarity arising from general similarities in the structure of the scale, and to compare the methods of reflectance in the two species. It is also hoped that the structure might give a clue as to how the sunlight-faded individuals have come to lose their UV reflectance so dramatically, as was also shown in Experiment 3-3.
The aim of this experiment is to determine whether or not the UV reflectance seen in white and yellow scales of Papilio dardanus is likely to have a structural origin, and if so to determine the mechanism by which the colours are produced, and how the reflectance may be lost with exposure to sunlight.
Small portions of wing were mounted flat in the electroconductive substance DAG on SEM stubs and sputter coated with gold. They were viewed in a Phillips 515 SEM. Individual scales were also sliced in half perpendicular to the longitudinal ridges under a microscope using a razor blade. These were then mounted on end, with the cut edge uppermost. The fine structure of the scales was photographed on monochrome Kodak T-Max 3200 film.
The following scales were mounted:
1) Coarse structure of the scales, as visible under magnification of approximately x200 to x500:
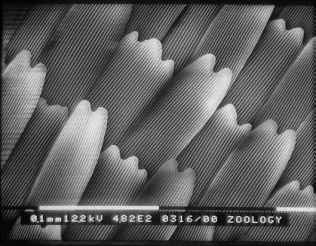
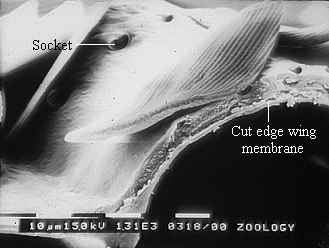
Figure 3-15 clearly shows the two types of scales present in Papilio dardanus - cover scales and ground scales. The cover scales are long and thin with two rounded lobes at the tip. These are interspersed amongst the slightly shorter and wider ground scales, which have three rounded lobes at the tip. Figure 3-16 demonstrates the same cover and ground scales in Danaus chrysippus. The cover scales are again slightly longer than the ground scales, although not as long as the scales in Papilio dardanus, and are simply rounded at the tips (there are no lobes present). The ground scales are also shorter than those of Papilio dardanus, and have four lobes at the tip (occasionally the central two lobes merge into one). In the top right hand corner, some scales have been displaced, and their sockets on the upper wing membrane are visible.

2) Fine structure, as visible with a magnification of x3500:
a)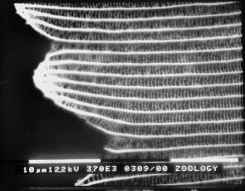 | b) |
The longitudinal ridges on the scales of Danaus chrysippus and Amauris niavius can be seen to be joined by simple crossribs (see Figure 3-17). In Danaus chrysippus the ridges (measured using Manostat calipers accurate to 0.05mm) are approximately 1.59Ám apart (all exactly the same within the accuracy of the measuring technique). In Amauris niavius the spacing between the ridges is approximately 2.00Ám (again all exactly the same).
a)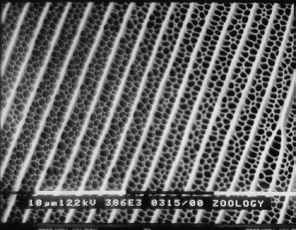 | b)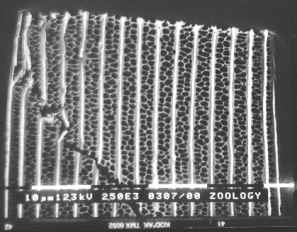 |
The ridges on the scales of Papilio dardanus can be seen to be joined by a net-like reticulum (see Figure 3-18), as would be expected in a Papilionid butterfly (Ghiradella, 1985). In the orange scales the ridges are all approximately 2.09Ám apart, and the diameter of the holes in the net is relatively large. In the white scales the ridges are all about 3.12Ám apart, and the holes are similarly sized.
a)  | b)  |
c)  | d)  |
In yellow scales from any race the ridges all have a spacing of about 2.73Ám, on both upper and lower wing surfaces (see Figure 3-19). The holes in the net appear to be smaller than in the other coloured scales. There was no obvious difference between the yellow scales from young Papilio dardanus individuals and the faded yellow scale from an old individual. In the black scales the ridges are approximately 2.46Ám apart, and the net holes appear to be similar to those on the yellow scales.
The mounting of scales with their cut ends visible proved to be very difficult, and of those that were successfully mounted, no focused image could be obtained of the cut end, owing to the vibration of such a thin sample in the beam of electrons (see Figure 3-20).
Although the SEM technique did not allow a focused image of the lamellae to be obtained, the general scale structures of Papilio dardanus, Amauris niavius and Danaus chrysippus could be compared. Papilio dardanus, as expected, showed a different scale structure from the other two species, illustrating the typical Papilionid reticulum form (Ghiradella, 1985). Differences between scales of different colours were also noted, although it is very unlikely that these differences will have any effect on the structural colours, as the variable distances - between the ridges, and the diameter of the holes in the reticulum - are far larger than the wavelength of light in the near UV and Visible regions of the spectrum (200-700nm), and thus will not cause any interference effects.
Therefore, to answer the questions posed in the introduction to this experiment, Transmission Electron Microscopy, in which the sample is fixed in resin and then sliced very thinly, is necessary as this prevents the sample from vibrating in the electron beam.
Transmission Electron Microscopy (TEM) allows ultrathin slices of material, fixed in resin, to be viewed at very high magnification. The technique has already been used successfully to investigate butterfly scale structure (e.g. Ghiradella et al., 1972). It can give a very clear image of a slice through the scale, and will reveal whether or not structures are present which would lead to the production of structural colours. This study should therefore reveal whether or not the UV reflectance measured in Experiment 3-3 can be attributed to structural reflectance or not.
In addition, if the structural colours are formed by the method of thin-film interference, as outlined in the introduction to Experiment 3-3, then it should be possible to measure the widths and spacings of the structures forming the layers from the TEM images, and thus use a mathematical model of thin-film interference (see introduction to Experiment 3-3) to predict whether or not the structure seen is consistent with the reflectance measured in Experiment 3-3 (as done by Ghiradella et al., 1972). However, the slices are very difficult to prepare and cut, and if measurements are to be taken of the layer-forming structures, care needs to be taken that the slices are exactly perpendicular to the basal membrane of the scale (a diagonal cut will give artificially enlarge measurements, importantly those of the width and spacings of the layer-forming structures).
TEM images of the yellow/orange scales of an old individual of Papilio dardanus may also suggest how the loss of UV reflectance seen in Experiment 3-3 arises because if the reflectance is structural in origin then its disappearance is likely to be due to a degradation of the reflecting structures.
The aim of this experiment is to determine the method of structural colour formation in Papilio dardanus, using a mathematical model, if possible, to test whether or not the structure observed is likely to result in the colours produced.
Small sections of wing were cut and placed in 1% osmium to stain for 1 hour. They were then put through a series of alcohol concentrations (70%, 80%, 95% and absolute), being in each for 30 minutes, to desiccate them. After that they were placed in 'absolute dry alcohol' (absolute alcohol over sodium sulphate), followed by a 50:50 solution of absolute alcohol and epoxypropane (propylene oxyde) and then one hour in pure epoxypropane. The sections were left overnight in a 50:50 mixture of araldite and epoxypropane with a lid on. The next day the mixture was allowed to vaporise before the sections were embedded in araldite. The araldite blocks were left in an oven overnight at 60(C, and then left to cool. Ultrathin slices were taken with a diamond Reichert-Jung Ultracut onto 400 bar grids. The slices were left to stain in saturated uranyl acetate for 10 minutes and then lead citrate for 5 minutes, and then viewed using a Philips EM 400 transmission electron microscope.
The following scales were examined:
It was interesting to see that the ridges both Papilio dardanus and Amauris niavius had the same reflective structure, despite the differences in the gross structure of the scales as seen under the SEM in Experiment 3-3. Photographs were taken under a magnification of 28,000 and measurements of the lamellae and air spaces taken off prints enlarged 5 times. A piece of software called Multilayer (Version 1.1, produced by Randy Geels) was then used to model thin-film interference (see Land, 1972 for a mathematical explanation of the process). The accuracy of the software was tested by predicting the structural colours in Eurema lisa and Morpho rhetenor using data from papers by Ghiradella et al. (1972) and Bingham et al. (1995) respectively, and found to be reasonable.
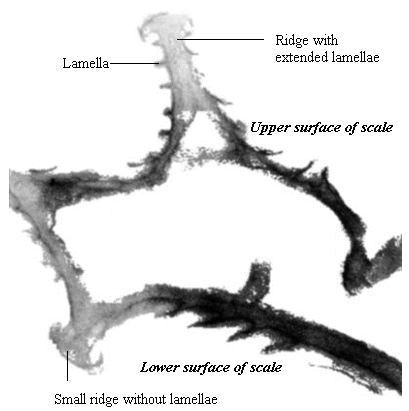
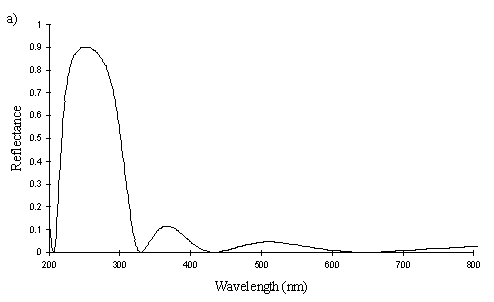
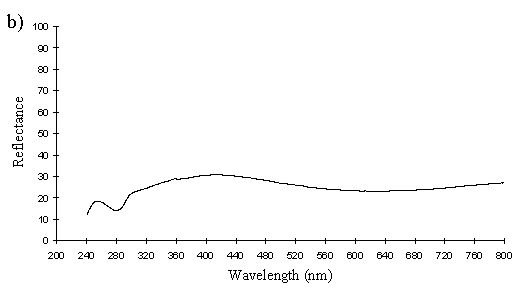
A cross section of a white Papilio dardanus hippocoonides scale clearly shows the tall ridges exhibiting the classic 'Christmas Tree' structure of ridges causing lamellar thin-film interference (see Figure 3-3) on the upper surface in contrast to the small, mushroom-shaped ridges carrying no structural reflectors on the lower surface (see Figure 3-21). The inside of the scale appears completely hollow, indicating that the colour of these scales is entirely due to reflectance, and that no pigment is present. Measurements of the widths of the lamellae and air spaces were taken off one side of one ridge which was best in focus. There are, on average, 5 complete lamellae on each side of the ridges (average taken from counting the lamellae on 6 sides), each with an mean width of 0.0297Ám ▒ 0.0052Ám (n=5) and with an average air space between them of 0.0821Ám ▒ 0.0097Ám (n=4). These values give a predicted major reflectance peak of 252nm, using Multilayer. Figure 3-22a shows the Multilayer prediction, with the very large primary peak indicating the structural colour predicted (the smaller peaks show negligible reflectance at other wavelengths). Figure 3-22b shows a typical spectrum obtained in Experiment 3-3 for the white patches in hippocoonides, illustrating the small UV peak at 255nm (the rest of the spectrum being due to pigmental reflection). The Multilayer prediction from the measurements of the lamellae matches this almost exactly, suggesting that this small peak visible in the reflectance spectrum is indeed caused by the lamellae acting as a thin-film interference filter.
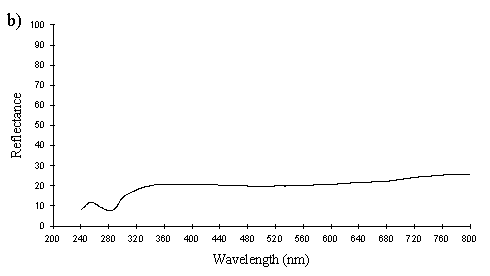
The cross sections of white scales from Amauris niavius (see Figure 3-23) show essentially the same structure as those of Papilio dardanus, with tall ridges and a series of exaggerated lamellae. It is interesting to note, however, that the interior of the scale is not hollow, as seen in hippocoonides (Figure 3-21). It is therefore possible that there is some pigmentation in the white scales of Amauris niavius. There are about 5 complete lamellae on each side of the ridges (average taken from counting the lamellae on seven complete sides of ridges), with an mean width of 0.039Ám ▒ 0.006Ám (n=33). The air spaces are an average of 0.083Ám ▒ 0.017Ám wide (n=28). Multilayer predicts a maximum reflectance of 286nm using these figures, which is slightly higher than that obtained experimentally (around 255nm). This difference would be caused by a slight overestimate of the width of the lamellae in the model. In the case of Amauris niavius the lamellae were very difficult to measure as the photographs taken were slightly out of focus in all cases. The resulting blur around the edges of the lamellae therefore probably caused the lamellae to be recorded as slightly wider than they actually are. However, it is still close to that obtained for hippocoonides (see Figure 3-24 and Figure 3-22) and it seems very likely that in both cases the measured UV reflectance peak is caused by the lamellae on the ridges acting as thin-film interference filters.

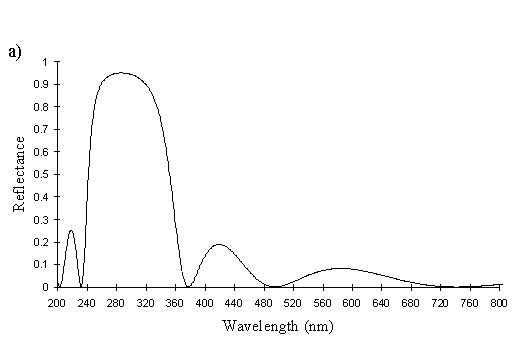


The yellow scales from a male Papilio dardanus (see Figure 3-25) are very similar to the white scales, with about 6 complete lamellae on each side of the ridges. Since the lamellae and the spaces between them increase down the ridge, for an accurate average measurement of the widths, an average was taken of the measurements of all the lamellae and air spaces from two complete sides. Although the sample size is therefore small, measuring the lamellae from only the top or bottom of the other ridges would bias the result one way or the other. The lamellae have an average width of 0.042Ám ▒ 0.015Ám (n=13) and the air spaces between them an average width of 0.084Ám ▒ 0.018Ám (n=12). Using Multilayer, this gives a prediction of a maximum reflectance around 300nm, which is close to that measured experimentally, around 322nm (see Figure 3-26). The interior of the scale is also not hollow as in hippocoonides (see Figure 3-21), but instead filled with what are presumably pigment granules containing Papiliochrome II. Again it is possible to see small ridges on the underside of the scale without structural reflectors. This difference between the yellow scales and white scales of hippocoonides means that the scales in hippocoonides have been structurally altered in order to produce the UV reflectance, which matches that found in Amauris niavius.

One scale from a faded yellow male Papilio dardanus shows possible damage to the lamellae on one side (see Figure 3-27). The lamellae are bent or broken on one side of the ridge, and this is seen on most of the ridges on the scale viewed. Although the evidence from only one scale is weak, this damage to the lamellae may be the explanation for the loss of UV reflectance in the faded specimens.

The orange scales of the yellow lamborni morph of Papilio dardanus have ridges which are markedly different from those in the white and yellow scales (see Figure 3-28 compared with Figure 3-21 and Figure 3-25). They are much shorter, and more widely spaced, with only an average of 3 lamellae, and these lamellae are much thicker - averaging 0.114Ám (0.039Ám wide (n=10), with air spaces of 0.133Ám (0.030Ám (n=7). These measurements produce a Multilayer prediction of no UV reflectance (see Figure 3-29) - the predicted peak in the visible spectrum is relatively low and shallow, rather than being sharply defined and with a high intensity as seen in the other predictions, and so is unlikely to cause any measurable reflection even in this region of the spectrum.

The black scales (taken from a young male Papilio dardanus) (see Figure 3-28) show a great difference in the structure of the ridges from the other scales investigated. The ridges are identical on both the upper and lower surfaces of the scale. They are all squat and mushroom-shaped with only the occasional protruding lamella. This means that they cannot act as thin-film stack reflectors. The interior of the scale is hollow, as melanin is usually distributed throughout the structure, rather than being sequestered into granules (Ghiradella, 1984).
The microstructure of the scales gives an interesting insight into the evolution of the species and its mimicry. The measurements of the number and widths of the lamellae allow a good prediction of the structural colours of the scales to be made when included in a thin-film interference model of reflectance (slight differences between the predicted and measured wavelengths are almost certainly due to the difficulties of accurate measurement of the lamellae and air spaces). Therefore it can be concluded that it is indeed these lamellae which cause the peaks seen in the UV region of the reflectance spectra. The yellow scales in Papilio dardanus have a different UV peak (322nm) from the white scales in the female hippocoonides morph (255nm), whilst the UV peak in hippocoonides exactly matches that measured in its model, Amauris niavius, and this can be seen to be due to a slight narrowing of the widths of the lamellae on the scale ridges. There is a lively debate as to how the different morphs evolved, and from what primitive condition. Trimen's original (1868) suggestion that the species was originally monomorphic, with both males and females showing the pattern now illustrated by males (as is the case in the monomorphic island races humbloti and meriones) has been adopted by one camp (including Poulton, 1924; Ford, 1936; Clarke & Sheppard, 1963; Turner, 1963; O'Donald & Barrett, 1973; Clarke et al., 1985). Another group (including van Bemmelen, 1922; Bernardi, 1963; Vane-Wright & Smith, 1991), however, have not been happy with this view as the male pattern appears to be so far derived from the traditional swallowtail pattern, whilst the mimetic females illustrate many more of the primitive swallowtail elements (see Nijhout, 1991 for further details on the patterning). Recent mitochondrial DNA analyses comparing Papilio dardanus races tibullus, meriones, humbloti, and also Papilio phorcas, Papilio constantinus (thought to be its two sister species) and Papilio nobilis (a Papilio species with a superficially similar appearance to the males, not thought to be closely related)(Vane-Wright et al., in press) appear to indicate that humbloti is indeed the most primitive race, although it is stressed that this result is only tentative, and further analyses, including more races, are ongoing. However, if the yellow patterning is indeed ancestral to the hippocoonides morph, then the white coloration has evolved not simply through the loss of Papiliochrome II in the yellow areas, but also by an adjustment of the size of the lamellae to produce an exact mimicry of the reflectance spectrum of its model Amauris niavius. The fact that the white in white lamborni has exactly the same reflectance spectrum of those in hippocoonides also appears either to have involved the same alteration of the scale structure from the yellow scales found in yellow lamborni (if the white lamborni morph is derived from yellow lamborni), or, as appears more likely from this evidence, the white lamborni morph evolved directly from hippocoonides, inheriting the scale structure of the white patches. If the latter scenario is correct then the evolution of the yellow lamborni morph requires an explanation.
It is interesting that the orange scales have lamellae, but these do not produce any reflection. In this way they are very different from the black scales. It is possible that they have evolved relatively recently from yellow scales (or white scales), and have not yet lost their lamellae completely. Whether they will or not depends on the cost of producing these lamellae which do not reflect.
The faded yellow scale shows the possibility that lamellae become damaged when exposed to sunlight, thus causing the loss in UV reflectancy seen in Experiment 3-3. If this is so, then we would also expect the white scales to lose their UV reflectance. This has yet to be studied. However, a larger number of scales of each colour need to be investigated to allow any firm conclusions to be drawn. The accuracy of measurements would also be greatly improved if an electron microscope with a digital display were used. This would allow accurate measurements to be taken directly from the sample. The difficulty of preparation precluded a greater range of individuals and scales being investigated in this study.
The previous experiments in this chapter have provided an objective assessment of what colours are actually present on the wings of Papilio dardanus (and its models). However, it is now necessary to measure what colour information the butterflies actually receive, before behavioural colour choice experiments can be interpreted.
Electroretinograms (ERGs) have been used to ascertain the visual sensitivities of many lepidoptera (see Experiment 3-3). The usual way to represent these is by plotting the spectral efficiency function - a graph of the electrical response of the retina to different wavelengths of light at constant intensity (quantum flux). By ascertaining the visual range of Papilio dardanus, and to which colours the butterfly is particularly sensitive, it should be possible to assess how the different morphs might appear to the butterflies themselves. This may also may help to explain any preferences for particular morphs the males might show (investigated in Chapter 5), as some morphs may appear brighter and more obvious to them, or preferences shown for particular flower colours (studied in Chapter 4). As discussed in Experiment 3-3, ERGs are usually carried out using a 500W xenon lamp, and using filters to bring the quantum flux for each wavelength down to the level of the lowest intensity wavelength. However, as pointed out by Menzel (1979, p507), the intensity function at each wavelength may be very different (i.e. the neuronal response could be non-linear with respect to intensity and different at each wavelength), and therefore the efficiency spectra usually plotted are only meaningful for the intensity at which they are recorded. In order to produce a more useful 'response surface' to assess the butterflies visual sensitivities at a range of light intensities, the response of butterflies would have to be measured at different intensities for each wavelength.
The aim of this experiment is to measure, by use of an ERG, the visual sensitivities of Papilio dardanus to different wavelengths and intensities of light, and to use this, together with the reflectance spectra of the morphs obtained previously, to assess the relative colours of the different morphs as seen by other individuals. The information will also be used in Chapter 4, which is a study of the colour preferences of Papilio dardanus with respect to flowers.
The wings were removed from an adult specimen of Papilio dardanus, and the insect was inserted into a tight plastic tube to prevent any movement of the limbs or antennae. Only the eyes protruded from the end of the tube. Two thin silver wire electrodes were then inserted just below the surface of one eye - one near the dorsal surface of the eye and the other nearer the ventral surface. The tube containing the butterfly was placed within a metal Faraday cage to shield the electrodes from electrical noise. A single beam of light could be allowed to enter the cage from a 200W mercury lamp. The light was passed through a lens containing continuously flowing water to remove the far Infra Red wavelengths and prevent overheating. The light was then passed through a Spectral Energy GM252 monochromator and finally focused onto the butterfly's eye with a silicon lens. The potentials from the recording electrode were passed through a Isleworth preamplifier (amplified x1000 with no frequency cuts) and to both a Tektronix Type 502A dual-beam oscilloscope and a Lloyd Instruments PL3 chart recorder.
The insect was dark-adapted for at least 5 minutes, and was then given brief flashes of monochromated light at 25nm intervals (+/- 2nm) between 200 and 850nm. 'Runs' of flashes between these wavelengths were repeated 5 or 6 times for each individual, and the procedure was repeated for 8 individuals in total (5 males and 3 females). Since the wavelength responses of the butterfly are due to a complex interaction between the response of the light-sensitive pigments and the fine structure of the eye (Menzel 1979, p518), and are therefore not predictable, it was important to repeat the procedure on a number of individuals and take an average result to obtain as accurate a result as possible.
This procedure gave the responses of the retina to light of the highest intensity which could be produced by the mercury lamp (much higher than a corresponding xenon lamp). However, the intensity of a mercury lamp is by no means constant across the spectrum, so this was measured using a photodiode, RS stock number 305-462 (RS Electronics *) (see Appendix 2 for further details). A set of filters (numerical swatch set, Lee Filters**) was then calibrated using the photodiode at the peak wavelengths of the lamp (375nm, 450nm, 550nm), as discussed in Experiment 3-3. The response of four individual butterflies was thus recorded in the same way to differing intensities of these three wavelengths. Since these showed that there was little or no variation in the intensity functions of different individuals, and that the data formed smooth curves with little noise, the surface was constructed using multiple readings from only one individual. This had the advantage that the butterfly was not moved throughout the series of readings, and so the same proportion of the beam was falling on the eye at all times from the lamp. If different individuals had been used, inevitable variations in the way they were mounted in the beam would have caused differences in the illumination of the eye by the beam and hence of the received intensities, and the readings from different individuals would have been shifted up or down the intensity curve slightly relative to one another by unknown amounts (see Appendix 2 for full details).
The wave-form of a lepidopteran ERG is described as a monophasic negative response (Eguchi et al. 1982). This is seen as a characteristic 'spike' above background noise in response to light stimulation:
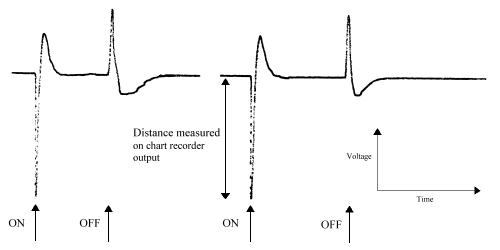
Occasionally an initial positive response is seen, which is a response from the optic ganglion layers (Swihart & Gordon 1971, Eguchi et al. 1982), but this was ignored. The mean response of each individual insect to each wavelength was calculated (over the number of runs carried out).
All the individuals, both males and females, showed a very similar spectrum of sensitivity to wavelength, but on varying scales of amplitude due to the differing placements of the electrodes and of the illuminating beam relative to the eye. The individual spectra were therefore normalised with respect to each other. This was done by selecting one spectrum (the one with the largest amplitude), and calculating the ratio of each point in an individual spectrum to the respective point in the selected spectrum. The mean ratio between each spectrum and the selected spectrum could then be calculated. Each point in each individual spectrum was then multiplied by this mean ratio for the particular spectrum to scale all the spectra with respect to that selected. Then a mean spectrum for all individuals was calculated. To avoid biasing the result due to the initial selection of one spectrum to which to normalise all the others, this mean spectrum was then used as the 'selected spectrum' and the process iterated until a true mean spectrum was achieved.
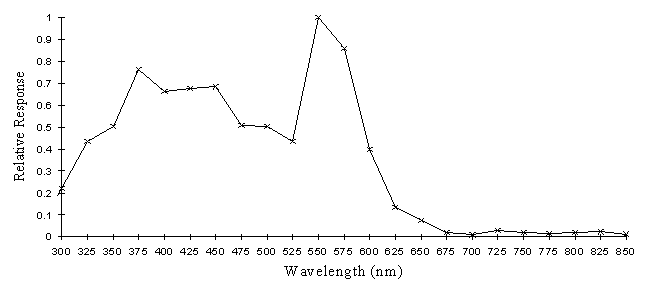
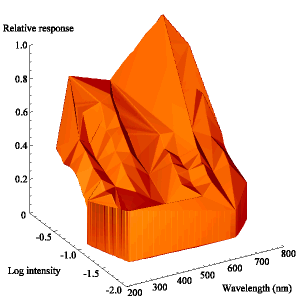
The intensity sensitivity functions were all relatively smooth, gentle curves when plotted on a log scale (see Appendix 2). It was thus relatively easy to calculate the spectral efficiency function for Papilio dardanus at a constant (low) intensity (see Appendix 2 for details) - see Figure 3-33. It was also possible to plot the raw data as a 'sensitivity surface', plotting the relative response at varying intensities for each wavelength (see Figure 3-44), and to plot the intensity sensitivity functions (calculated in Appendix 2) for each wavelength to create an apparent surface (see Figure 3-45).
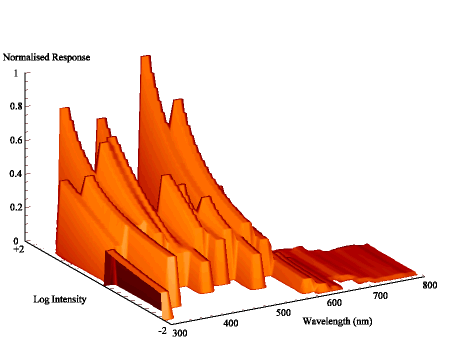
The spectral efficiency function for Papilio dardanus (at a constant intensity) shows peaks at 350nm (UV), 475nm (blue), 525nm (green), and 575nm (orange). These compare well with those reported for Papilio xuthus (Arikawa et al., 1987) which were found at 360nm, 400nm (no corresponding pigment found in Papilio dardanus), 460nm, 520nm, and 600nm (the Papilio dardanus peak in this area appears to be shifted slightly toward orange). The three main visual pigments found in insects are present, at approximately 360nm, 460nm, and 520nm (Chittka & Menzel, 1992; Backhaus & Menzel, 1992; Bennett et al., 1997; Bernard & Remington, 1991).
How this measured output from the retina is interpreted by the butterflies is a matter of debate. In true 'colour vision', the output from the receptors containing visual pigments would be compared with each other, and the perceived colour would be a weighted average of the intensity received by each. Hence humans perceive purple when red and blue receptors are stimulated, and the perceived purple tends to red as comparatively more red or less blue is received. The alternative method of interpreting the output of the receptors would be for the butterflies to show 'wavelength-specific behaviour', where the receptor outputs are independently assessed, and each simply triggers a certain behaviour once it reaches a threshold of stimulation. Scherer & Kolb (1987) performed experiments designed to test for the presence of true colour vision in the butterflies Aglais urticae and Pararge aegeria. They found that the butterflies did indeed appear to have true colour vision (although they also showed wavelength-specific behaviour). Therefore it seems likely that Papilio dardanus would have similar colour information processing, and this experiment indicates that they would have at least as good a colour vision as humans in the Visible range of the spectrum, and also possess good UV sensitivity.
The peaks in the Visible spectrum correspond to the wavelengths reflected well by Papiliochrome II (445-800nm), but the UV peak does not quite correspond to the peak reflectance by Papiliochrome II (which is about 320nm) (See Figure 3-31). The butterflies will, however, still be able to detect the UV component well, and so the overall colour of the pigment will appear slightly shifted toward the blue end of the spectrum to them, compared with how we perceive it which make make it less obvious against the vegetation, as discussed in Experiment 3-3. The reflectance spectra of the white patches on hippocoonides (and Amauris niavius) show high reflectivity over all the spectrum between 360nm-800nm, and therefore will appear true white to the butterflies, and distinguishable from the background vegetation by the presence of reflectance in the red/orange region of the spectrum as well as by a difference in brightness. The orange pigment has a reflectance spectrum which corresponds very well with the upper peaks in sensitivity, and has no UV component, so will appear reddish to the butterflies, also contrasting in hue from the background. The intensity-wavelength sensitivity surfaces (plotted in Figure 3-34 and Figure 3-35) show the general trend of greater response to greater intensities, but as the data is very incomplete (due to the need for higher intensities at certain wavelengths than the lamp was able to produce) it is difficult to interpret.
The results of this experiment will be very important in interpreting any colour preferences indicated by work in Chapter 4 (on the colour preferences of Papilio dardanus whilst feeding) and Chapter 5 (on mate choice in Papilio dardanus).
Cook et al. (1994) described how the older males and trimeni females start to fade to an orange colour, apparently due to the action of sunlight, and Cockayne (1924) described how the yellow pigment in Papilionids "readily changes to a browner tint and becomes non-fluorescent". In Experiment 3-3 the colour change was demonstrated to involve a loss of green reflectance as well as fluorescence, and in addition the scales tended to lose their UV reflectance (which was shown to be a structural phenomenon in Experiment 3-3). Umebachi (1961) named the yellow pigment Papiliochrome II (which occurs together with very small quantities of a pigment he named Papiliochrome III), one of the Papiliochrome pigments unique to the Papilionid butterflies. The pigment is said to be broken down by heat and weak ammonia solution (Umebachi & Yoshida, 1970), but there is no description of chemical change under the action of light.
The fact that older individuals may gradually change colour introduces the interesting possibility of an age-cue in the species (Cook et al., 1994). This might be used by females in choosing a mate. A preference for younger males, judged by the extent of wing damage, has been found in Colias eurytheme (Rutowski, 1985), and this could be explained as a way of choosing more fertile partners as it has been shown in swallowtails that ejaculate mass decreases very rapidly in males (Svńrd & Wicklund, 1986).
It would be very interesting to do mate choice experiments to investigate whether or not females discriminate between males on the basis of their colour, and in order to do this with live males, it would be necessary to accelerate the fading process to compare males which differ in their colour but not in their actual age (so as not to give any other cues to the females which might affect their choice). If the fading of the pigment is caused by UV light, of which there is comparatively little in sunlight (Robinson, 1966) it might be possible to accelerate this process using an Ultraviolet lamp which produces a much higher intensity of the wavelengths responsible for the fading.
The aim of this experiment is to identify the wavelengths which cause the colour change of Papiliochrome II.
The wings of freshly dead male Papilio dardanus were pinned out in a glass greenhouse (to protect them from rain and wind). In each case one wing was completely protected from light, and another wing was half covered by a filter. The wings were left until the area completely exposed to the sunlight showed distinct fading (38 days between the 18th May and 25th June 1998). Both wings were then scanned together, using a HP Deskscan II, to ensure the same brightness and contrast settings.
Five different filters were used:
- A UV filter (Image Optics Components*** ) which removed all wavelengths shorter than 400nm (see Figure 3-37).
- A red filter (Lee Filters**) which removed all wavelengths shorter than 575nm (see Figure 3-39).
- A yellow filter (Lee Filters) which removed all wavelengths shorter than 475nm (see Figure 3-38).
- A green filter (Lee Filters) which removed wavelengths between 600 and 700nm and had limited transmission to other wavelengths (see Figure 3-41).
- A blue filter (Lee Filters) with a similar spectrum, but slightly higher transmission to other wavelengths (see Figure 3-40).
(Spectra for red, yellow, green and blue filters obtained from manufacturer, spectra for UV filter obtained using UV-Vis spectrometer)

Figure 3-38 The transmission spectrum of the yellow filter
Figure 3-39 The transmission spectrum of the red filter
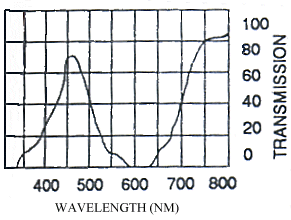
Figure 3-40 The transmission spectrum of the blue filter
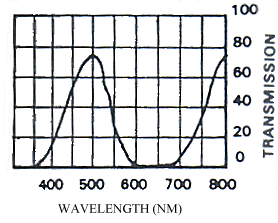
Figure 3-41 The transmission spectrum of the green filter
1) The UV filter did not prevent fading of the pigment (see Figure 3-42, filtered region of right wing), therefore the UV component of sunlight cannot be responsible for the process. The very small difference in colour between the exposed and filtered parts of the right hand wing could be due to the fact that the filter also removes about 5-10% of the intensity of all other wavelengths (see Figure 3-37).
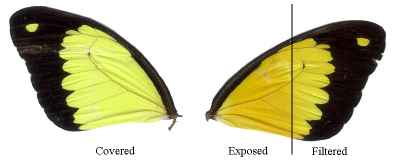
2) The filter removing wavelengths shorter than 575nm appeared to prevent fading altogether (see Figure 3-43). This indicates that the wavelengths responsible for the fading are shorter than 575nm, but the result from the UV filter indicate that they are longer than 400nm.
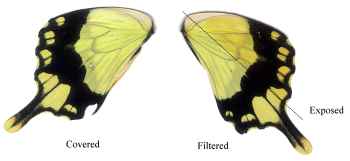
3) The filter removing wavelengths shorter than 475nm also prevented any fading from occurring (see Figure 3-44). This indicates that the wavelengths responsible for the change are shorter than 475nm, and it is already known from the result of applying a UV filter that they are longer than 400nm.
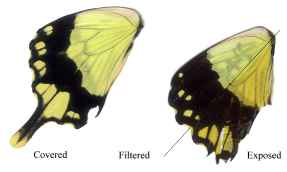
4) The green filter, as would be expected from the results above, allowed some fading of the pigment (see Figure 3-45)
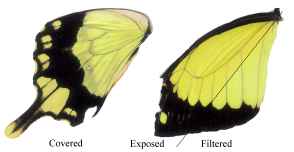
5) The blue filter, with a transmission spectrum which was virtually identical to the green filter in the region 400-475nm, gave a very similar result to the green filter as might be expected, and the two are not distinguishable (see Figure 3-46).
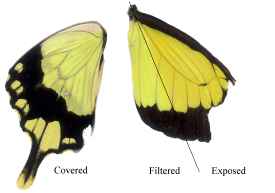
It can therefore be seen that removing the wavelengths shorter than 400nm (UV) has no effect on the fading of Papiliochrome II, while removing the wavelengths shorter than 475nm (green/blue/UV) prevents its fading. It can therefore be concluded that the wavelengths responsible are between 400 and 475nm. This results in some fading being seen with the blue and green filters which allow some limited transmission in these regions.
This experiment indicates that the wavelengths responsible for the colour change in the yellow Papiliochrome II pigment of Papilio dardanus lie between 400 and 475nm, and are therefore in the blue/violet rather that the UV region of the spectrum. It is therefore not possible to cause artificially rapid fading in live butterflies by using a UV bulb in order to create 'apparently old' males for use in mate choice experiments. If a bulb with a suitably strong illumination in the 400-500nm region of the spectrum could be used to fade live specimens, mate choice experiments using them could provide very interesting data on whether or not the females use the colour change of older males as an age-cue when selecting mates. Similar experiments could be carried out on the effect that such illumination of andromorphic females had on male choice. Since such a bulb could not be found, these experiments could not be carried out.
The photographs from Experiment 3-1 demonstrate that the patterning of the morphs of Papilio dardanus is likely to be unchanged by different spectral sensitivities of the signal receivers. The reflectance spectra from Experiment 3-3 (and the knowledge, from Experiment 3-2, that the fluorescence of the yellow pigment is so relatively weak) indicate how the colours might be seen by receivers with varying sensitivities, and the ERG data from Experiment 3-6 gives an idea of the sensitivities of the butterflies themselves, and hence allows an assessment of the signals they might be receiving from each other. If the butterflies have true colour vision, as has been shown in other species (Scherer & Kolb, 1987) the UV reflectancy of the yellow scales would shift the perceived colour of the patches slightly toward the blue end of the spectrum for the butterflies compared with how they appear to us, and thus the spectral contrast between the wings and the non-UV reflectant vegetation (Klein, 1978) could be much lower than we perceive it to be. This means that the yellow need not necessarily be obvious to the butterflies themselves. The true-white patches of hippocoonides, however, are likely to be very obvious against the background vegetation. The orange colour, having no UV component, should appear in relatively the same area of the spectrum to the butterflies as it does to us.
The reflectance spectra show that the white patches in the hippocoonides morph appear to have exactly the same reflective properties (including the UV range) as those in Amauris niavius, increasing the close resemblance of the model and mimic. The UV peak of the white patches in both species is 255nm, as opposed to one of 320nm found in the yellow patches of Papilio dardanus (assumed to be the ancestral colour). The transmission electron microscopy has shown that this is due to a structural change in the width and spacing of the lamellae on the scale ridges. This demonstrates that hippocoonides is quite a sophisticated mimic of Amauris niavius, and that the mimicry was not due simply to a loss of yellow pigment from a male-like trimeni form. The fact that there appears to be evolutionary pressure for the hippocoonides to mimic Amauris niavius in this extreme range of the spectrum indicates that the main predators probably have spectral sensitivity in this region, and this should be investigated further.
The role of the orange pigment is a matter for debate. It seems that it has either appeared independently in both hippocoonides- and trimeni-like forms, or that it occurred firstly in a trimeni-like form, and that the white, trophonius morph is subsequently derived from the yellow, prototrophonius morph which has not yet been totally excluded by the presence of the trophonius form. There are two hypotheses to explain the presence of the lamborni morphs. Cook et al. (1994) suggested that for females trying to avoid 'sexual harassment' (which they suggest as a reason for the evolution of a male-mimicking trimeni form), there might be a selection pressure for loss of fluorescence and reflectance in order to mimic the older males (which they demonstrated to be approached even less often by males than younger males). However, this hypothesis does not explain why the orange pigmentation is so strictly limited to the hindwings and lower forewings - fully orange forms such as pale poultoni (found in race polytrophus from Kenya) would be much more likely mimics of faded males. It also does not explain the presence of white lamborni (trophonius) - why should a hippocoonides-like morph evolve an orange pigmentation over part of its wings, leaving white flashes on the forewings, disrupting its mimicry of Amauris niavius, and not being a good mimic of a faded male Papilio dardanus? The spectral similarity of the white patches in white lamborni and hippocoonides and the fact that this appears to be due to an alteration of the structure of the scales suggests that the white lamborni form is derived directly from hippocoonides, although without studying the spectrum of the white patches in Danaus chrysippus it is not possible to say that hippocoonides and white lamborni have not independently evolved the same coloration.
Some mainland trophonius morphs do resemble Danaus chrysippus very closely (Trimen, 1869), and it seems very likely that these are indeed mimics of Danaus chrysippus. According to the second hypothesis (Trimen, 1869 and subsequent authors) the yellow lamborni (prototrophonius) evolved first from a trimeni-like morph due to predator pressure causing a resemblance of Danaus chrysippus (although this first stage could have been due to a 'sexual harassment' pressure as suggested by Cook et al.). Following the evolution of yellow lamborni (prototrophonius), the evolution of white lamborni (trophonius) would simply be due to a pressure for a closer mimicry of Danaus chrysippus. The similarity of the spectra of hippocoonides and white lamborni appears to run counter to this proposed sequence of events, but as has been mentioned previously, the spectra of Danaus chrysippus need to be studied before all the evidence can be assessed. Whatever the pressures involved, the presence of apparently non-functional lamellae in the orange scales of lamborni suggest that the orange pigment may have evolved relatively recently, and the fact that the lamborni or trophonius/prototrophonius morphs are dominant over all other morphs in the races in which they occur also suggest that they might have appeared most recently. It has been suggested that dominance over existing mimetic forms evolves in new mimetic forms (Sheppard, 1967), and a mathematical analysis of the situation has confirmed that in many cases this will be true, as a poorer mimic (as the first individuals of a new mimetic form will almost certainly be) will tend to become dominant over a better mimic of another model (Charlesworth & Charlesworth, 1976). This result, however, is based on many assumptions, and cannot be held as a strong prediction, so the patterns of dominance within Papilio dardanus can only be regarded as suggestive of the patterns of evolution of the morphs.
The fact that the faded individuals (which would be older in life than those which had not had a chance to fade) had become very much less fluorescent and, more importantly, less UV reflectant (shifting their apparent colour towards red), means that they are likely to be clearly perceptible as older to potential mates. This may therefore be a mechanism by which females can choose between potential partners (see the introduction to Experiment 3-4). However, any male mutant which managed to retain its reflectance longer would leave more offspring (by obtaining matings later in life, even given decreased fertility), so it would seem likely that some means of retaining the original coloration would evolve if at all possible if the selection pressure is large enough. It is also conceivable that females may choose an older partner, who has proved his survival ability (despite the fact that he may have a lower viability due to previous matings). Again, however, it might be expected that males would have 'cheated' and evolved an orange pigment, possibly similar to that found in the female pale poultoni morphs if females preferred such orange coloration. Mate choice experiments in this area are required, but as discussed in Experiment 3-4 it has proved very difficult to fade the males artificially, and hence males which are genuinely older than others would have to be used in order to gauge any preference. This will be discussed further in Chapter 5. However, the information gained from this chapter on the signals actually produced by the different morphs of Papilio dardanus, and its visual sensitivities will prove invaluable in interpreting the results of the next two chapters, on the flower colour preferences and mate choices of the species.
I would like to thank Sandor Ertz at the British Antarctic Survey, Cambridge for the use of the spectrophotometer; Richard Compton for the use of the fluorescence spectrometer, chart recorder, mercury lamp and monochromator; Jenny Corrigan and Martin Lomas for help with the preparation and viewing of the electron microscopy samples; and the Zoology Department Photographic Unit for the development and printing of the photographs. I am also very grateful to Steve Simpson and John Alden for help with the ERG measurements; and to Pauline Rigby for introducing me to the Multilayer software.
Anderson, T.F. & Richards, A.G. 1942. An electron microscope study of some structural colours of insects. J. Appl. Phys. 13, 748-58.
Arikawa, K., Inokuma, K., Eguchi, E. 1987. Pentachromatic visual system in a butterfly. Naturwissenschaften 74, 297-298.
Autrum, H. 1958. Electrophysiological analysis of the visual systems in insects. Expl. Cell Res. (suppl.) 5, 426-439.
Backhaus, W. & Menzel, R. 1992. Conclusions from the colour vision of insects. Behavioural and Brain Sciences 15 (1), 28-30.
Bennett, R.R., White, R.H., Meadows, J. 1997. Regional specialisation in the eye of the sphingid moth Manduca sexta: blue sensitivity of the ventral retina. Visual Neuroscience 14, 523-526.
Bernard, G.D. 1979. Red-absorbing visual pigment of butterflies. Science 203 (1), 1125-7.
Bernard, G.D. & Remington, C.L. 1991. Color vision in Lycaena butterflies: spectral tuning of receptor arrays in relation to behavioural ecology. Proc. Natl. Acad. Sci. USA 88, 2783-2787.
Bernardi, G. 1963. Quelques aspects zoogÚographiques du mimÚtisme chez les LÚpidpidoptŔres. Proceedings, 16th International Congress of Zoology, Washington 4, 161-166.
Bingham, L., Bingham, S., Geary, S., Tanner, J., Driscoll, C., Cluff, B., Gardner, J.S. 1995. SEM Comparison of Morpho butterfly dorsal and ventral scales. Microscopy Research and Technique 31, 93-4.
Brunton, C.F.A. & Majerus, M.E.N. 1995. Ultraviolet colours in butterflies - intraspecific or interspecific communication? Proc. Roy. Soc. Lond. B 260 (1358), 199-204.
Carpenter, G.D.H. 1941. Observations and experiments in Africa by the late C.F.M. Swynnerton on wild birds eating butterflies and the preference shown. Proc. Linn. Soc. Lond. 154, 10-46.
Charlesworth, D. & Charlesworth, B. 1976. Theoretical genetics of Batesian mimicry III Evolution of dominance. J. Theor. Biol. 55, 325-337.
Chen, D-M. & Goldsmith, T.H. 1986. Four spectral classes of cone in the retinas of birds. J. Comp. Physiol. A 159, 473-479.
Chittka, L. & Menzel, R. 1992. The evolutionary adaptation of flower colours and the insect pollinators' colour vision. J. Comp. Physiol. A 171, 171-181.
Clarke, C.A., Clarke, F.M.M., Collins, S.C., Gill, A.C.L., Turner, J.R.G. 1985. Male-like females, mimicry and transvestism in swallowtail butterflies. Systematic Entomology 10, 257-283.
Clarke, C.A., Gordon, I.J., Vane-Wright, R.I., Smith, C.R. 1991. Phylogenetic relationships of three African swallowtail butterflies, Papilio dardanus, P. phorcas and P. constantinus: new data from hybrids (Lepidoptera: Papilionidae). Systematic Entomology 16, 257-273.
Clarke, C.A. & Sheppard, P.M. 1963. Interactions between major genes and polygenes in the determination of the mimetic patterns of Papilio dardanus. Evolution 17, 404-413.
Cook, S.E., Vernon, J.G., Bateson, M., Guilford, T. 1994. Mate choice in the polymorphic African swallowtail butterfly, Papilio dardanus - male-like females may avoid sexual harassment. Animal Behaviour 47 (2), 389-397.
Cockayne, E.A. 1924. The distribution of fluorescent pigments in the Lepidoptera. Trans. Entomol. Soc. London, 1-19.
Eguchi, E., Watanabe, K., Hariyama, T., Yamamoto, K. 1981. A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. J. Insect Physiol. 28 (8), 675-682.
Ford, E.B. 1936. The genetics of Papilio dardanus Brown (Lep.). Transactions of the Royal Entomological Society of London 85. 435-466.
Ghiradella, H., Aneshansley, D., Eisner, T., Silberglied, R.E., Hinton, H.E. 1972. Ultraviolet reflection of a male butterfly: Interference color caused by thin-layer elaboration of wing scales. Science 178, 1214-1217.
Ghiradella, H. 1974. Development of ultraviolet-reflecting butterfly scales: How to make an interference filter. Journal of Morphology 142, 395-410.
Ghiradella, H. & Radigan, W. 1976. Development of butterfly scales, 2: Struts, lattices, and surface tension. Journal of Morphology 150, 279-298.
Ghiradella, H. 1984. Structure of iridescent lepidopteran scales: Variations on several themes. Annals of the Entomological Society of America 77, 637-645.
Ghiradella, H. 1985. Structure and development of iridescent lepidopteran scales: The Papilionidae as a showcase family. Annals of the Entomological Society of America 78, 252-264.
Ghiradella, H. 1989. Structure and development of iridescent butterfly scales: Lattices and laminae. Journal of Morphology 202, 69-88.
Ghiradella, H. 1991. Light and colour on the wing - structural colours in butterflies and moths. Applied Optics 30 (24), 3492-3500.
Ghiradella, H. 1994. Structure of butterfly scales: Patterning in an insect cuticle. Microscopy Research and Technique 27, 429-438.
Kayser, H. 1985. Pigments. In Comprehensive insect physiology, biochemistry and pharmacology Vol. 10 ed. Kerkut, G.A. & Gilbert, L.I., 367-415. Pergamon, Oxford.
Kevan, P., Giurfa, M., Chittka, L. 1996. Why are there so many and so few white flowers? Trends in Plant Sciences 1(8), 280-284.
Klein, R.M. 1978. Plants and near-ultraviolet radiation. Bot. Rev. 44, 1-127.
Land, M.F. 1972. The physics and biology of animal reflectors. Progress in Biophysics and Molecular Biology 24, 77-123.
Lippert, W. & Gentil, K. 1959. ▄ber lamellare Feinstrukturen bei den Schillerschuppen der Schmetterlinge vom Urania and Morpho-Typ. Z. Morph. Íkol. Tiere 48, 115-122.
Mason, C. 1926. Structural colours in insects, 1. J. Phys. Chem. 30, 383-395.
Mason, C. 1927a. Structural colours in insects, 2. J. Phys. Chem. 31, 320-354.
Mason, C. 1927b. Structural colours in insects, 3. J. Phys. Chem. 31, 1856-1872.
Menzel, R. 1979. Spectral sensitivity and colour vision in invertebrates. In Handbook of Sensory Physiology Vol. VII/6A "Comparative physiology and evolution of vision in invertebrates" ed. Autrum, H., 503-580. Springer, Berlin.
Nijhout, H.F. 1985. The developmental physiology of colour patterns in Lepidoptera. Advances in Insect Physiology 18, 181-247.
Nijhout, H.F. 1991. The development and evolution of butterfly wing patterns. Smithsonian Institute Press.
O'Donald, P. & Barrett, J.A. 1973. Evolution of dominance in polymorphic Batesian mimicry. Theoretical Population Biology 4, 173-192.
Poulton, E.B. 1924. Papilio dardanus. The most interesting butterfly in the world. Journal of the East African and Ugandan Natural History Society 20, 4-22.
Robinson, N. 1966. Solar Radiation. Amsterdam, Elsevier.
Rutowski, R.L. 1985. Evidence for mate choice in a sulphur butterfly (Colias eurytheme). Z. Tierpsychol. 70, 103-114.
Scherer, C & Kolb, G. 1987. The influence of color stimuli on visually controlled behavior in Aglais urticae L. and Pararge aegeria L. (Lepidoptera). J. Comp. Physiol. A 161, 891-898.
Sheppard, P.M. 1967. Natural selection and heredity (3rd edn.). Hutchinson, London.
Silberglied R.E. 1979. Communication in the Ultraviolet. Ann. Rev. Ecol. Syst. 10, 373-398.
Svńrd, L. & Wiklund, C. 1986. Different ejaculate delivery strategies in first versus subsequent matings in the swallowtail butterfly Papilio machaon L.. Behav. Ecol. Sociobiol. 18, 325-330.
Swihart, C.A. 1971. Colour discrimination by the butterfly, Heliconius charitonius Linn. Anim. Behav. 19, 156-164.
Swihart, S.L. & Gordon, W.C. 1971. Red photoreceptor in butterflies. Nature 231, 126-127.
Trimen, R. 1869. On some remarkable mimetic analogies among African butterflies. Transactions of the Linnean Society of London 26, 497-522.
Turner, J.R.G. 1963. Geographical variation and evolution in the males of the butterfly Papilio dardanus Brown (Lepidoptera: Papilionidae). Transactions of the Royal EntomologicalSociety of London 115, 239-259.
Umebachi, Y. 1961. Yellow pigments in the wings of the papilionid butterflies - V. Some chemical properties of the yellow pigments of Papilio xuthus. Sci. Rep. Kanazawa Univ. 7, 139-150.
Umebachi, Y. & Yoshida, K. 1970. Some chemical and physical properties of Papiliochrome II in the wings of Papilio xuthus. J. Insect Physiol. 16, 1203-1228.
Van Bemmelen, J.F. 1922. The wing-design of mimetic butterflies. Proceedings, Section of Sciences, Koninklijke Nederlandse Akademie Wetensschappen 23, 877-886.
Vane-Wright, R.I., Raheem, D.C., Cieslak, A., Vogler, A.P. In press. Evolution of the mimetic African swallowtail butterfly Papilio dardanus: molecular data confirm relationships with P. phorcas and P. constantinus. Biol. J. Linn. Soc.
Vane-Wright, R.I. & Smith, C.R. 1991. Phylogenetic relationships of three African swallowtail butterflies, Papilio dardanus, P. phorcas, and P. constantinus: a cladistic analysis (Lepidoptera: Papilionidae). Systematic Entomology 16: 275-291.
|
Please cite this thesis as: Freeman, ALJ; 1998; D.Phil thesis, Oxford University. |
E-mail to Alexandra Freeman Back to Table of Contents Back to Home Page |